Author: Exploring the bacteria release date: 2018-07-16
The efficient and precise genetic modification of human T cells without the need for viral vectors is the latest breakthrough in the journalism published by scientists. The "Magic Clip" CRISPR has played a vital role in it. Researchers believe that this achievement is of great significance in the fields of scientific research, medicine and industry, and will greatly accelerate the development of next-generation cell therapy and bring new hope for the treatment of diseases such as cancer.
Image source: Network
Viruses cause infection by injecting their own genetic material. Since the 1970s, scientists have begun to use this feature of the virus: the use of viral vectors to transport DNA to cells for research, gene therapy, and so on.
However, building viral vectors is a very difficult and expensive process, and the shortage of clinical-grade vectors has also led to production bottlenecks in gene therapy and cell therapy. In addition, even if available, viral vectors are far from an ideal tool because they insert genes into the genome of the cell at will, which can damage existing health genes or prevent newly introduced genes from ensuring normal cell functioning. Control of the adjustment mechanism. These limitations can lead to serious side effects and have raised concerns about gene therapy and cell therapy such as CAR-T.

Image source: Nature
Get rid of the new technology of "virus"
On July 11th, in a study published in the journal Nature entitled "Reprogramming human T cell function and specificity with non-viral genome targeting", scientists from the University of California, San Francisco (UCSF) developed a type that was not used. The virus can be "rewritten" with the powerful technology of the human T cell genome.
In particular, the technique relies on electroporation: applying an electric field to the cells such that the cell membrane temporarily has greater permeability. After a year of exploration, the team led by Dr. Alex Marson of UCSF found that when a certain number of T cells, DNA and CRISPR were mixed together and then exposed to an appropriate electric field, T cells were able to accurately integrate specific gene sequences into the genome. The site that was cleaved by CRISPR.

Alex Marson, MD, PhD, UCSF immunologist whose group has developed an approach to edit T cell genomes without using viruses, speaks with MD/PhD student Theo Roth. Credit: Noah Berger.
In fact, members of Dr. Marson's lab have previously achieved some degree of success in using electroporation and CRISPR insertion of small amounts of genetic material into T cells. However, a large number of experiments attempting to insert long DNA sequences into T cells failed: leading to cell death. This has led many researchers to believe that large (long) DNA sequences are too toxic to T cells.
To demonstrate the versatility and strength of the new technology, Dr. Marson used it to repair a disease-causing gene mutation in T cells and create "custom T cells" that can find and kill human melanoma cells.
According to Theodore L. Roth, the first author of the paper, scientists have been trying to insert new genes into T cells for 30 years. In their study, after nearly a year of trial and error, Roth determined the ratio of T cell number, DNA number, and CRISPR abundance, combined with an electric field with appropriate parameters to achieve efficient and accurate T cell genome. Editor.
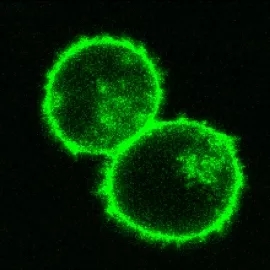
The research team created CRISPR guides that would cause green fluorescent protein to be expressed in only certain cellular locations and structures. Credit: Alex Marson's Lab.
"strength" verification
In the study, Roth first designed CRISPR to label a different set of T cell proteins with green fluorescent protein (GFP). The results confirmed that the new technology is highly specific and the off-target effect level is very low: GFP is only specific. Expression in cell location and structure.
Then they began to verify the therapeutic prospects of the new technology. In the first experiment, Roth and colleagues used T cells supplied to Dr. Marson's lab by Kevan Herold of Yale School of Medicine. These cells are from three siblings with rare, severe autoimmune diseases. Genomic sequencing revealed that these children's T cells carry mutations in the IL2RA gene. The IL2RA gene encodes a cell surface receptor that is critical for the development of regulatory T cells (Tregs, which inhibit other immune cells and prevent autoimmunity).
Using a new CRISPR-based technology, the UCSF team quickly repaired IL2RA deficiency in childhood T cells, as well as cellular signals damaged by mutations. The researchers hope that, like the anti-cancer using CAR-T therapy, similar technologies can effectively treat autoimmune diseases.
In the second experiment, with the new technology, scientists completely replaced the "natural T cell receptors in normal human T cells" with "new receptors specifically designed to recognize specific subtypes of human melanoma cells." Cell receptors, sensors used to detect diseases and infections." In culture dishes, engineered T cells show specificity: not only can the target melanoma cells be effectively found, but other cells are also ignored. This is very important for the precise treatment of cancer.
The researchers emphasized that they could produce a large number of engineered cells that were engineered to express new T cell receptors without the use of viral vectors. When these CRISPR-engineered cells were transplanted to mice carrying human melanoma, they were able to enter the tumor site and showed anticancer activity.
Set of test tubes in Alex Marson's lab. Credit: Noah Berger.
Broad prospects
Roth said that it has changed the research environment of Dr. Marson's lab because the new technology made it possible to "create a custom T cell line available in a little more than a week." Previous experiments with viral vectors were now available.
“This is a fast, flexible way to change, enhance, and reprogram T cells. Just as fast as speed and ease of use, new technologies have made 'injecting large numbers of DNA fragments into T cells'. Possibly, this will give T cells powerful new features," Dr. Marson commented.
In summary, the researchers hope that their new technology, a fast, versatile, and economical approach based on CRISPR, can be widely used in emerging cell therapy to accelerate the development of new, safer cancers, autoimmune Treatment of diseases and other diseases, including rare genetic diseases.
References: 1) T Cell Engineering Breakthrough Sidesteps Need for Viruses in Gene-Editing - With Faster, Cheaper, More Precise Technique, Authors Say It's 'Off to the Races' Toward New Cell Therapies
Original title: Nature heavy: faster, cheaper, more accurate! "Magic Clip" CRISPR or rewrite cell therapy
Source: Bio-Exploration
Chinese Gallnut Extract also known as Chinese gall or nutgalls. It is a plant excretion produced when irritants are released by the larvae of gall insects, such as those of the Cynipidae family, the gall wasps. Oak trees are the major commercial source of medicinal gallnuts.
1. With the function of antioxidant and antimicrobial;
2. With the function of treating chronic diarrhea and dysentery
3. With the function of enhancing immunity.
4.It has the effect on improving the body disease-resistant ability, liver and gastrointestinal systemfunction;
5. With the function of dispelling heat, diuretic, eliminating irritability, cooling blood and detoxification;
Gallnut Extract,Gallnut Extract Powder,Organic Gallnut Extract,Natural Gallnut Extract
Allied Extracts Solutions , https://www.alliedadditives.com