Release date: 2018-02-12
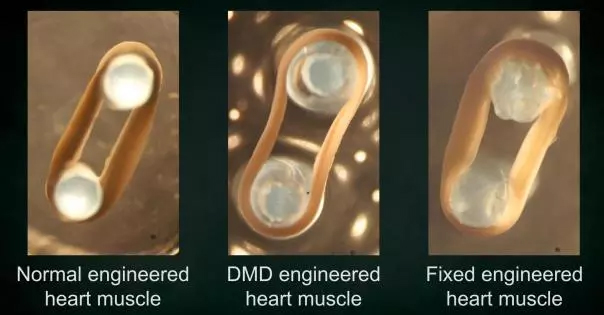
Scientists from the University of Texas Southwestern Medical Center (UT) recently published a research report that they used a CRISPR/Cas9 gene editing technology to correct most of the 3,000 genetic mutations that cause Duchenne muscular dystrophy (DMD). mutation. In a proof-of-concept study, Dr. Eric Olson and colleagues used single-cut myoediting to successfully restore normal dystrophin production in cells derived from DMD patients and carrying a range of different DMD gene mutations. . In 3D engineered heart muscle, myoediting of DMD mutations successfully restored the expression of dystrophin and its contractile force in cardiomyocytes.
According to reports, this new method requires single-cutting at specific sites in the DMD gene and eliminates the need to develop a separate gene editing tool for each DMD mutation. Dr. Olson said that this is a very important advancement, and we hope that this technology will ultimately alleviate the pain and even save lives of patients with DMD who carry a wide range of mutations.
The study was recently published in Science Advances entitled "Correction of Diverse Muscular Dystrophy Mutations in Human Engineered Heart Muscle by Single-Site Genome Editing (with single-point genome editing to correct various DMDs in human engineered muscles) Gene mutation)".
The incidence of DMD in men is 1 in 5,000, a genetic disease caused by mutations in the X-linked dystrophin gene. The DMD gene has 2.6 million base pairs and is one of the largest known genes in the human genome. Its size and complex structure result in a very high rate of spontaneous mutation. To date, up to 3,000 DMD gene mutations have been documented, including large fragment deletions or duplications, small fragment deletions, and point mutations.
Dr. Olson's team and other research teams have previously used CRISPR/Cas9-mediated genome editing techniques to correct DMD gene point mutations in mouse models. However, as the researchers point out, most DMD patients carry a mutation in one or more exon deletion mutations, while only 5%-9% of DMD patients carry a point mutation. Given that thousands of mutations have been identified in humans, one obvious question is how such a large number of mutations can be corrected by CRISPR/Cas9-mediated genome editing techniques.
In fact, most DMD genes are mutated into clusters that are concentrated in the "hotspot" region of the gene (exons 45 to 50, and exons 2 to 29). This means that skipping one or two of the 12 target exons (called top 12 exons) located in or near the "hot spot" and thus eliminating the abnormal splice sites can be practically A functional dystrophin is produced in 60% of patients with DMD. In this study, the team created “myoediting†to correct DMD gene mutations by exon skipping.
In the study, the team screened a guide RNA that would direct the Cas9 enzyme to skip the first 12 exons of the DMD gene and restore a functional dystrophin production. The goal was to develop a CRISPR/Cas9 tool that works on group mutations, so skipping a single exon restores dystrophin expression in most patients.
In the study, the team applied optimized guide RNA, using myoediting technology to induce pluripotent stem cells from cardiomyocytes derived from DMD patients and presenting a variety of DMD gene mutations (including large deletions, point mutations, sequence repeat mutations, etc.) In (iPSC), the production of dystrophin was restored.
In addition, detection using 3DEHM also found that the use of a single guide RNA to myoediting DMD gene mutations also successfully restored dystrophin expression and muscle contractility. Fortunately, the study found no need to correct the production of dystrophin in each cardiomyocyte in the myocardial model, but only correcting a group of cardiomyocytes (30% to 50%) is enough to rescue the mutant EHM phenotype. Bring it close to the normal control level.
At present, scientists have proposed many different treatments, but myoediting provides a real hope for prolonging the lives of patients and improving their quality of life. Not only did we find a practical way to treat many mutations, but we also developed a less destructive method to skip defective DNA instead of removing them. The genome is highly structured and you don't want to delete DNA fragments that may be very important.
According to this study, the same single-cut CRISPR/Cas9 method can also be practically developed for the treatment of other diseases caused by single gene mutations. However, the precondition is that after the target exon is removed, the target gene must be able to function. Dr. Olson pointed out that dystrophin is like a shock absorber, and it is okay to remove a few turns of spring. But other proteins may not be that easy.
In February 2017, Dr. Olson founded the startup Exonics Therapeutics, which licensed the CRISPR/Cas9 technology from the UT Southwestern Medical Center to develop drugs for the treatment of DMD. Previously, the new company had received $5 million in seed financing from CureDuchenne Ventures.
Source: Sina Medicine (micro signal sinayiyao)
GMP Certificated Immune Globulin Injection Supplier in China
Hepatitis B Immunoglobulin,Hep B Immunoglobulin,Hepatitis B Immunoglobulin Vaccine,Hepatitis Immune Globulin
FOSHAN PHARMA CO., LTD. , https://www.full-pharma.com