1. Selection of stationary phase using ACE bonded phase 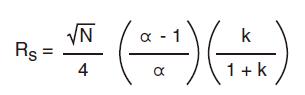
The resolution equation determines the parameters that affect the resolution: efficiency (N), capacity factor (k), and selectivity (α).
In the past few years, column efficiency and the use of ultra-high performance columns (called "UHPLC" columns) have been given great attention as a means of achieving separation targets.
UHPLC has proven its value through faster separation and faster method development to increase laboratory productivity.
However, selectivity is often overlooked and its importance is masked by the emphasis. This is regrettable.
Among the three parameters affecting the resolution, selectivity is the most important.
(See Figure 1) Better and faster separation is usually achieved by utilizing efficiency and selectivity.
Figure 1: Effect of N, α and k on resolution (Rs)
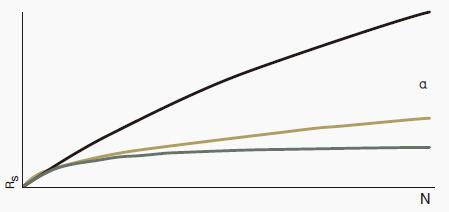
Increasing N, α or k can increase the resolution (Rs).
However, as can be seen from these figures, as the value of N or k is increased, the effect of improving the resolution is gradually lowered.
On the other hand, increasing the selectivity (α) does not have this problem, so it becomes the optimal optimization variable when developing the separation method.
In reversed-phase chromatography, there are multiple mechanisms of interaction between the stationary phase and the analyte, which can be used to achieve separation.
These interaction mechanisms include: hydrophobic binding, π-π, hydrogen bonding, dipole-dipole, and shape selectivity.
Different types of bonded phases will provide one or more of these interaction mechanisms. Table 1 lists the ACE bonded phases and each of the possible major interaction mechanisms, depending on the analyte and mobile phase conditions.
Table 1: Comparison of separation mechanisms/interactions of different bonded phases
Better separation is achieved by the selectivity of the bonded phase.
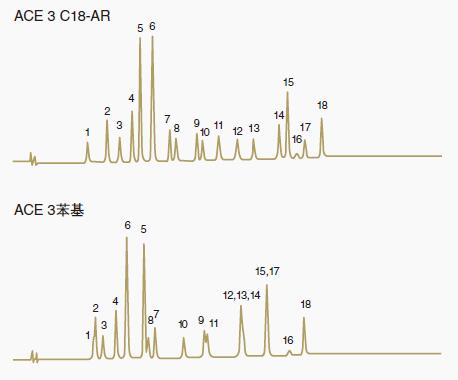
Figure 2 shows the selectivity difference between the two ACE bonded phases, C18-AR and phenyl.
Although the two bonded phases offer the possibility of strong π-π and dipole-dipole interactions, there are significant differences in the strength of other possible interaction mechanisms, particularly hydrophobic binding.
C18-AR may have other different separation mechanisms that provide better separation for complex mixtures. This may not be the case for other mixtures, which is an advantage of the ACE bonded phase.
When developing separation methods, the ACE bonded phase provides a variety of important retention mechanisms to choose from.
The very powerful and unique C18-AR and C18-PFP phases are available only on ACE and ACE Excel columns.
Better separation can be achieved by utilizing efficiency and selectivity.
Figure 2: Comparison of selectivity differences between C18-AR and phenyl phase
Column size: 50 x 2.1 mm, 3 μm
Mobile phase:
A = 20 mM KH2PO4, pH 2.7 (dissolved in water);
B = 20 mM KH2PO4, pH 2.7 (dissolved in methanol/water: 65:35, v/v)
Flow rate: 0.6 mL/min
Temperature: 60 °C
Detection: UV 214 nm
Gradient: From 3 to 100% B in 5 minutes for 1 minute.
sample:
Metronidazole
2. 3-hydroxybenzoic acid
3. Phenol
4. Benzyl alcohol
5. Caffeine
6. Salicylic acid
7. Quinoxaline
8. Benzoic acid
9. Quinine
10. phenacetin
11. 1,4-Dinitrobenzene
12. 1,3,5-trinitrobenzene
13. Furosemide
14. 1,3,5-trimethoxybenzene
15. Piroxicam
16. Carvedilol
17. Ethyl benzoate
18. Nortriptyline
The C18-AR phase is more hydrophobic, which provides more retention and better selectivity for chromatographic peak pairs (13, 14) and (15, 17). Also note that there are many variations in the elution order of C18-AR and phenyl phase.
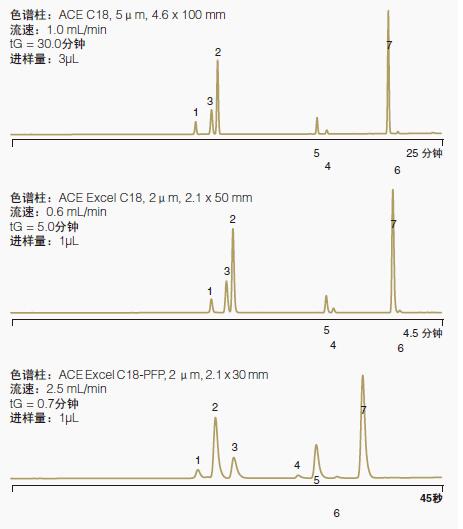
Figure 3 shows another example of the bonding phase selection capability.
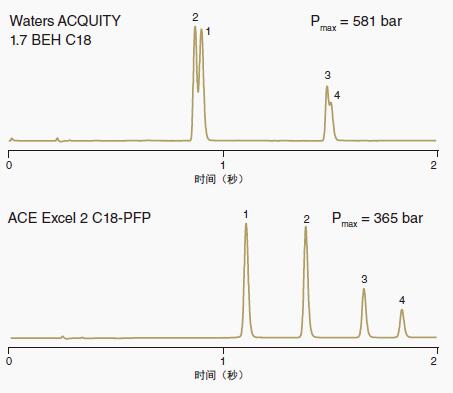
One separation was done with a UHPLC column with a C18 bonded phase and the other with a UHPLC column with a C18-PFP bonded phase.
The additional separation mechanism provided by the C18-PFP bonded phase makes the overall separation better.
Figure 3: ACE Excel for superior resolution and peak shape: UHPLC results for drugs and their related substances
Conditional column size: 50 x 2.1mm
Mobile phase:
A = 5 mM formic acid (dissolved in water)
B = 5 mM formic acid (dissolved in methanol)
Gradient: from 3 to 100% B in 5 minutes
Flow rate: 0.6 mL/min
Temperature: 40 °C
Detection: UV 254 nm
sample
Paracetamol
2. Hydrochlorothiazide
3. Methylphenyl sulfoxide
4. Methyl sulfone
Chromatograms were generated equally rapidly on C18 UHPLC columns and C18-PFP columns.
However, C18-PFP columns provide better selectivity for peak pair (13, 14) and (15, 17), thus providing superior overall separation performance.
2. The ACE and ACE Excel silica-based matrix stationary phases virtually eliminate the adverse effects of silanol groups on chromatographic separation and have earned a reputation for providing superior peak shapes for basic compounds.
Peak tailing reduces resolution, reduces sensitivity, and even interferes with accuracy and precision (Figure 4).
There are many potential causes for peak tailing, but the main reason for the separation of basic materials is the interaction between the acidic silanol groups on the surface of the silica stationary phase support particles and the amino groups on the analyte (Figure 5).
Figure 4: Effect of peak tailing on resolution and sensitivity
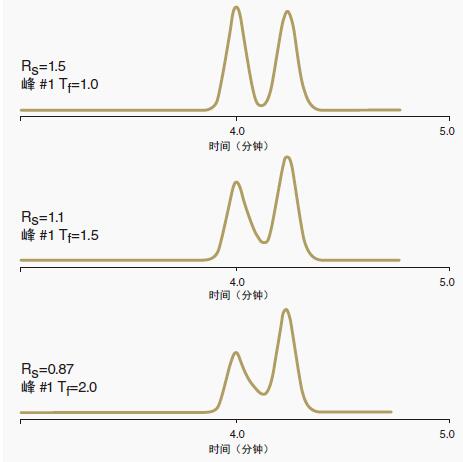
As the peak tail (Tf, tailing factor) increased from 1.0 to 2.0, the resolution (Rs) decreased from 1.5 to 0.87.
As the peak width increases and the peak area remains the same, the sensitivity (peak height) also decreases as the peak tail increases.
The ACE column is made of ultra-inert silica with very low silanol activity.
This ultra-pure silica gel uses patented technology for effective bonding and complete sealing.
The stationary phase leading to this silica matrix virtually eliminates the adverse effects of silanol groups on chromatographic separation.
The ultra-inert nature of the ACE column makes it ideal for the separation of polar basic compounds.
When separating complex basic compounds, ACE columns always give significantly better peak shape and efficiency than other modern alkaline deactivation columns (Figure 6).
Figure 5: Peak tailing interaction
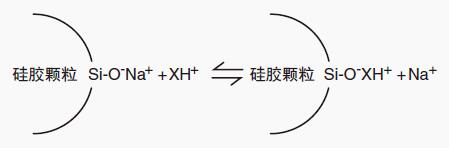
The acidic silanol groups on the surface of the silica stationary phase support form an ion exchange site that interacts with the basic compound.
This ion exchange interaction typically results in peak retention (secondary retention) and causes peak tailing when separating amine compounds using reverse phase HPLC.
 A silica-based stationary phase that almost eliminates the adverse effects of silanol groups on chromatographic separation
A silica-based stationary phase that almost eliminates the adverse effects of silanol groups on chromatographic separation
Figure 6: Peak tailing comparison condition Column size: 50 x 2.1 mm
Mobile phase: 40% methanol, 60% water flow rate: 0.2 mL/min
Temperature: 22oC
Sample: pyridine
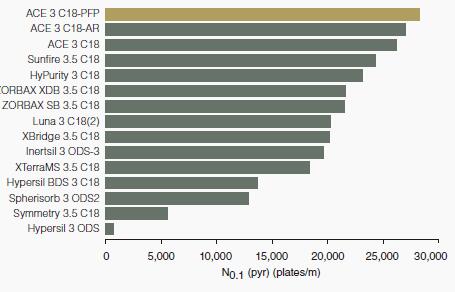
The number of plates of the basic compound (pyridine) on various common C18 columns is reported.
The number of plates was measured at 10% peak height to allow peak tailing to be included in the measurement.
ACE C18-PFP achieves the highest number of plates due to its ultra-inert nature.
3. ACE Excel brings the renowned ACE HPLC performance to UHPLC <br> It is important to recognize that column selectivity and efficiency are independent variables, you don't have to choose one when developing a separation method. Use another one. In fact, in order to achieve the best overall separation, you should optimize both variables and retention values. This is especially true when high speed separation is required. (See Figure 7)
Figure 7: Using a column efficiency and bonded phase selectivity to develop a faster separation method
condition:
Mobile phase:
A = 5 mM formic acid (0.189) ml / L;
A = 5 mM formic acid (dissolved in methanol)
Column temperature: 40 ° C
Detection: PDA, 254nm
sample:
Aspirin
2. phenacetin
3. 1,3-dinitrobenzene
4. Ethyl benzoate
5. Nimesulide
6. Ibuprofen
7. Indomethacin
With a higher efficiency on UHPLC columns, shorter columns can be used at higher mobile phase flow rates to reduce the separation time of this mixture by 80% (compared to HPLC columns).
However, the separation time can be further shortened by optimizing the selectivity of the bonded phase, in this case utilizing the enhanced selectivity of the C18-PFP bonded phase.
Compared to C18UHPLC columns and C18 HPLC columns, the ACE Excel C18-PFP UHPLC column reduces the separation time of the mixture by more than 80% and 96%, while maintaining excellent resolution of all peaks.
The ACE Excel UHPLC column is designed to take advantage of low-diffusion, ultra-high pressure UPLC® and UHPLC instruments (up to 1000 bar) and is compatible with all commercially available UPLC and UHPLC systems.
ACE Excel UHPLC columns provide better peak shape for basic compounds, improve column-to-column reproducibility, provide additional stationary phases that utilize multiple separation mechanisms, and improve column durability.
The availability of ACE Excel UHPLC columns means that chromatographers have more choices when using UPLC and UHPLC instruments for better results.
Figure 8: ACE Excel columns provide fast, high resolution separation conditions: ACE Excel C18, 2 μm, 3.0 x 50 mm
Mobile phase: from 30% B to 100% B in 60 seconds
A = water + 0.1% TFA B = acetonitrile flow rate: 2.5 mL / min
Column temperature: 40oC
Pressure: 703 bar (10,335 psi)
Detection: PDA, 254 nm
Instrument: Agilent 1290 UHPLC
Compound
Acetanilide
2. Acetophenone
3. Propiophenone
4. Phenylbutanone
5. Benzophenone
6. Phenylpentanone
7. Phenylhexanone
8. Benzheptanone
9. Benzophenone
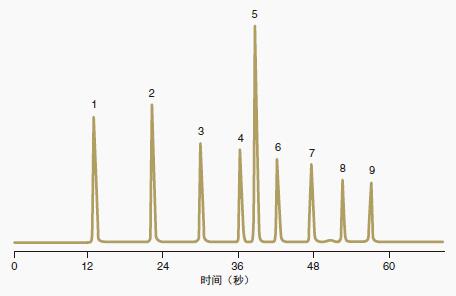
The ACE Excel C18 column provides baseline separation of these nine analytes in less than 60 seconds.
4. ACE HPLC columns and ACE Excel UHPLC columns have proven to be durable, reliable, and have excellent column life.
The acidified hydrolysis of the siloxane bonded to the silica stationary phase support can lose the bonded phase, thereby reducing the lifetime of the column. The acid hydrolysis increases as the pH of the mobile phase decreases and the column temperature increases.
Shorter alkyl phases such as C4 and C3 and the phenyl phase are generally more susceptible to loss of the bonded phase than the C18 phase. In addition, low purity silica gel stationary phase supports and low surface coverage stationary phase supports make some bonded phases more susceptible to acid hydrolysis.
The ACE bonded phase exhibits extremely high stability due to the use of ultra-high purity silica-immobilized carrier particles and extremely high surface coverage of the bonded phase. (See Figure 9)
Although all ACE bonded phases are excellent in stability, some bonded phases are not as stable as other bonded phases. For example, it is generally believed that the C18 bond is more stable than the alkyl phase, the PFP phase, and the phenyl bonded phase having a shorter column length.
In fact, poor stability of a typical phenyl stationary phase is one of the main reasons why this phase is not often selected in method development.
To solve this problem, ACE C18-AR was developed, which allows chromatographic analysis to utilize the powerful π-π and dipole-dipole separation mechanisms while still enjoying the robustness and durability of the C18 phase.
As shown in Figure 10, ACE C18-AR not only has very excellent stability to a typical phenyl bonded phase, it even exhibits better stability than many other C18 bonded phases.
Similarly, ACE C18-PFP provides a variety of separation mechanisms that can be used to achieve better overall separation while benefiting from the stability of the C18 phase.
Figure 9: Acid stability, pH 1.8
Conditional column: ACE 4.6 x 150 mm
Mobile phase: 50% CH3CN, 50% water flow rate: 1.0 mL/min
Temperature: 22 °C
Analyte: phenanthrene acid exposure conditions:
Mobile phase: 50% CH3CN 50% 0.1% TFA (dissolved in water) (pH 1.8)
Flow rate: 1.0 mL/min
Temperature: 22 °C
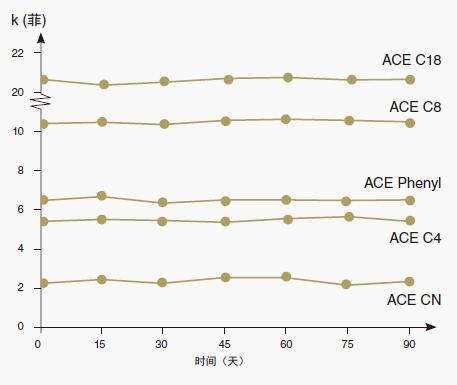
Figure 10: Acceleration column stability study: 80 ° C at pH 1.9
Acidic exposure conditions:
Mobile phase: 5:95 MeOH/0.1% TFA (dissolved in water) (pH 1.9)
Flow rate: 0.20 mL/min
Temperature: 80 °C
Column size: 50 x 2.1mm
Analyte: Philippine
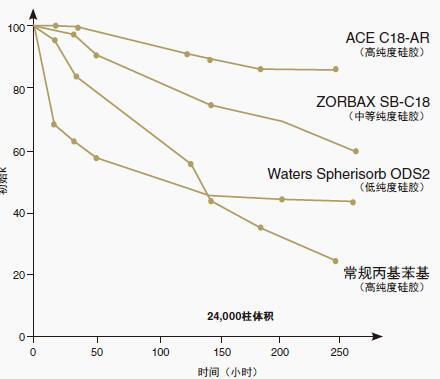
Using conditions designed to accelerate column degradation, the ACE C18-AR phase exhibits minimal retention losses and its lifetime corresponds to the highly stable ACE C18 phase. Both phases are made of the same ultrapure silica and are more durable than Zorbax SB-C18 (Zorbax SB-C18 was once considered a stationary phase with excellent stability at high temperatures and low pH) .
As expected, C18 bonded columns based on low purity silica gel (Waters Spherisorb ODS2) have a much reduced lifetime under these accelerated conditions.
Of particular note is the comparison of the lifetime of conventional phenyl columns with ACE C18-AR. Compared to ACE C18-AR, the lifetime of phenyl columns is reduced despite the use of high purity silica, suggesting that ACE C18-AR may be suitable for applications where phenylpropyl columns have a short lifetime.
It is quite common to attach HPLC and UHPLC to mass spectrometers commonly referred to as LC-MS.
However, mass spectrometry is very sensitive to traces of the bound phase (called column "bleed") that may elute from the HPLC/UHPLC column. Column bleed may interfere with the analytical results and, importantly, the column selected in the LC-MS application has a sufficiently stable bonded phase to avoid excessive column bleed.
Figure 11 shows that the ACE C18-PFP column has little "loss" interference with LC-MS analysis.
The stability of C18-PFP (and ACE C18 and ACEC18-AR) makes these columns more suitable for LC-MS applications.
Figure 11: Low column bleed of the ACE C18-PFP column makes it an ideal choice for LC/MS applications. Column size: 50 x 3.0 mm
Mobile phase: Gradient: 5 to 95% B in 5 minutes, 5 minutes in 95% B
A: 0.1% formic acid (dissolved in water)
B: 0.1% formic acid (dissolved in acetonitrile)
Flow rate: 0.43 mL/min
Temperature: 60oC
Detection: Agilent 1100 MSD ESI positive ion fast scan mode, fast scan all 50 – 1000 m/z
Sample: blank run
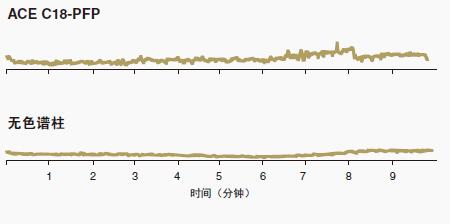
Column bleed can interfere with the detection and measurement of target analytes in LC-MS applications.
Column loss was not shown on the ACE C18-PFP column.
The low bleed characteristics of all ACE columns make them ideal for LC-MS applications.
UHPLC columns may lack durability. Coupled with the possibility of blockage of the inlet screen, you may face column durability that is not as problematic as an HPLC column.
The ACE Excel column is very tolerant and offers the same durability and longevity you expect from an HPLC column.
ACE Excel will change your view of the durability of UHPLC columns.
The ACE Excel column is not only better packed, but also features a patented inlet screen design that makes it less susceptible to blockage than other UHPLC columns.
It is still recommended that you filter the sample and mobile phase as you would any UHPLC column, but the ACE Excel column is more tolerant than other UHPLC columns and offers the same durability and longevity as HPLC columns.
Figure 12: ACE Excel columns have a good reputation for durability
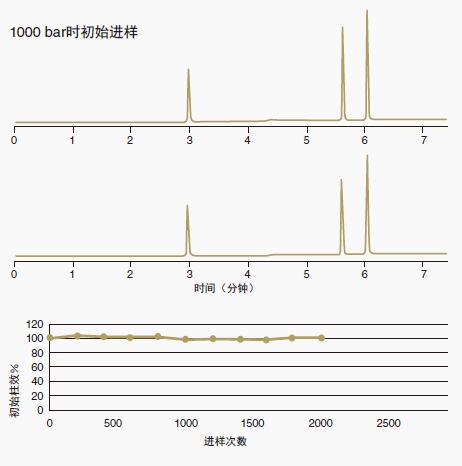
A 2.1 x 100 mm ACE Excel C18 UHPLC column was run over 2000 gradients at an average pressure of 1,000 bar (14,500 psi).
At the end of the stability study, the efficiency (plate number) and retention time remained essentially unchanged.
5. ACE Excel columns have excellent reproducibility.
The reproducibility between the column and the column is affected by the formation of the silica stationary phase support, the binding of the stationary phase to the stationary phase support, and the filling of the stationary phase in the column.
During the preparation of the column, the better each stage is controlled, the better the reproducibility and quality of the column.
The ACE column is fully controlled at every stage of the column preparation process.
These controls ensure that ACE columns produce reliable and predictable performance between columns and between batches.
Figure 13 provides an example of a typical batch verification test.
Subtle changes in silanol activity are one of the main reasons for the selective change between the column and the column.
ACE and ACE Excel columns offer excellent reproducibility due to the use of ultra-pure reagents and rigorous manufacturing process control (high purity silica stationary phase media with uniform surface characteristics).
Combining this high-purity silica with advanced bonding technology produces a range of highly inert stationary phases that virtually eliminate silanol interference in the chromatogram, providing superior column-to-column weight Present.
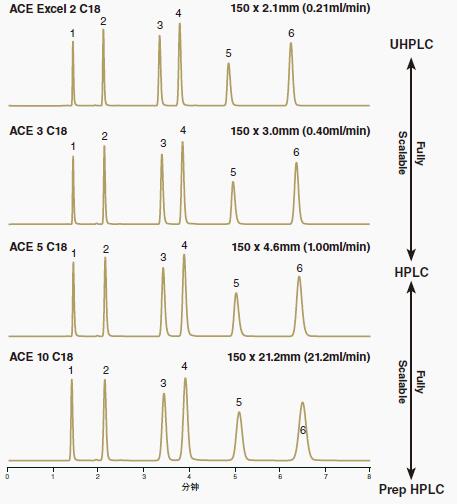
6. ACE columns provide easy scalability from UHPLC to HPLC to preparative HPLC.
The same quality control ensures high reproducibility of different batches, and also makes it easier to convert between UHPLC and HPLC methods, or to scale up to preparation applications when separating and purifying target compounds.
The efficiency of the column will of course change, but it is expected that the band spacing (selectivity) will be the same.
Figure 14 illustrates that when the same ACE bonded phase but different particle size packed columns are used for operation, the analyte-consistent band spacing type (selectivity) can be foreseen.
Figure 14: Selectivity retention and assurance when the ACE stationary phase was extended from UHPLC (2 μm) to HPLC (3 μm and 5 μm) to preparative HPLC (10 μm).
Conditional mobile phase: 35:65 MeCN/0.1% TFA (dissolved in water)
Temperature: 22 °C
Wavelength: 254 nm
Analyte
Uracil
2. 4-hydroxybenzoic acid
3. Acetylsalicylic acid
4. Benzoic acid
5. 2-hydroxybenzoic acid
6. Ethyl p-hydroxybenzoate
These chromatograms of the test samples were run on the same column with the same bonded phase but different particle sizes, and the results elucidated the ease with which the separation was extended from UHPLC to HPLC to preparative HPLC.
When using ACE Excel UHPLC columns for rapid method development, and the method must be transferable to HPLC, ease of scalability is particularly valuable.
Figure 15: ACE stationary phases provide the same selectivity, whether they are UHPLC, HPLC or preparative HPLC column conditions: 2.1 x 50 mm
Mobile phase: from 30% B to 90% in 1.4 minutes B
A = 20 mM ammonium formate + 0.1% formic acid
B = acetonitrile + 0.1% formic acid gradient: 0.30 minutes at 30% B, 30 to 90% B in 1.10 minutes, 0.40 minutes at 90% B Flow rate: 0.5 mL/min
Column temperature: 30oC
Detection: AB Sciex Triple Quad(TM) 5500 MS, operating in ESI (+) mode: Shimadzu Prominence UFLC System Analyte
Mebendazole
Proprolin
3. Amitriptyline
4. Loperamide

These chromatograms of the test samples were run on the ACE Excel C18 2 μm U HPLC column, ACE C18 3 μm HPLC column, and ACE C 185 μm column with the same mobile phase conditions. The results demonstrate that the selectivity of the ACE stationary phase is consistent regardless of the particle size of the fill.
This makes the transfer of methods from UHPLC to HPLC or HPLC to UHPLC easier. It also makes it easier to extend from analytical applications to preparative applications.
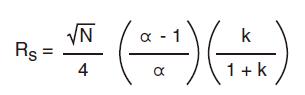
The resolution equation determines the parameters that affect the resolution: efficiency (N), capacity factor (k), and selectivity (α).
In the past few years, column efficiency and the use of ultra-high performance columns (called "UHPLC" columns) have been given great attention as a means of achieving separation targets.
UHPLC has proven its value through faster separation and faster method development to increase laboratory productivity.
However, selectivity is often overlooked and its importance is masked by the emphasis. This is regrettable.
Among the three parameters affecting the resolution, selectivity is the most important.
(See Figure 1) Better and faster separation is usually achieved by utilizing efficiency and selectivity.
Figure 1: Effect of N, α and k on resolution (Rs)

Increasing N, α or k can increase the resolution (Rs).
However, as can be seen from these figures, as the value of N or k is increased, the effect of improving the resolution is gradually lowered.
On the other hand, increasing the selectivity (α) does not have this problem, so it becomes the optimal optimization variable when developing the separation method.
In reversed-phase chromatography, there are multiple mechanisms of interaction between the stationary phase and the analyte, which can be used to achieve separation.
These interaction mechanisms include: hydrophobic binding, π-π, hydrogen bonding, dipole-dipole, and shape selectivity.
Different types of bonded phases will provide one or more of these interaction mechanisms. Table 1 lists the ACE bonded phases and each of the possible major interaction mechanisms, depending on the analyte and mobile phase conditions.
Table 1: Comparison of separation mechanisms/interactions of different bonded phases
| ACE Bonded phase | Hydrophobic bonding | ––π | Hydrogen bonding | Dipole-dipole | Shape selectivity |
| C18 | Strong | no | no | no | weak |
| C18-HL | Extremely strong | no | no | no | weak |
| C18-AR | Strong | Strong | medium | medium | medium |
| C18-PFP | Strong | Strong | Strong | Strong | Strong |
| AQ | medium | no | medium | weak | no |
| C8 | medium | no | no | no | no |
| C4 | weak | no | no | no | no |
| Phenyl | medium | Strong | weak | medium | weak |
| CN | weak | no | weak | Strong | no |
Better separation is achieved by the selectivity of the bonded phase.
Figure 2 shows the selectivity difference between the two ACE bonded phases, C18-AR and phenyl.
Although the two bonded phases offer the possibility of strong π-π and dipole-dipole interactions, there are significant differences in the strength of other possible interaction mechanisms, particularly hydrophobic binding.
C18-AR may have other different separation mechanisms that provide better separation for complex mixtures. This may not be the case for other mixtures, which is an advantage of the ACE bonded phase.
When developing separation methods, the ACE bonded phase provides a variety of important retention mechanisms to choose from.
The very powerful and unique C18-AR and C18-PFP phases are available only on ACE and ACE Excel columns.
Better separation can be achieved by utilizing efficiency and selectivity.
Figure 2: Comparison of selectivity differences between C18-AR and phenyl phase

Column size: 50 x 2.1 mm, 3 μm
Mobile phase:
A = 20 mM KH2PO4, pH 2.7 (dissolved in water);
B = 20 mM KH2PO4, pH 2.7 (dissolved in methanol/water: 65:35, v/v)
Flow rate: 0.6 mL/min
Temperature: 60 °C
Detection: UV 214 nm
Gradient: From 3 to 100% B in 5 minutes for 1 minute.
sample:
Metronidazole
2. 3-hydroxybenzoic acid
3. Phenol
4. Benzyl alcohol
5. Caffeine
6. Salicylic acid
7. Quinoxaline
8. Benzoic acid
9. Quinine
10. phenacetin
11. 1,4-Dinitrobenzene
12. 1,3,5-trinitrobenzene
13. Furosemide
14. 1,3,5-trimethoxybenzene
15. Piroxicam
16. Carvedilol
17. Ethyl benzoate
18. Nortriptyline
The C18-AR phase is more hydrophobic, which provides more retention and better selectivity for chromatographic peak pairs (13, 14) and (15, 17). Also note that there are many variations in the elution order of C18-AR and phenyl phase.
Figure 3 shows another example of the bonding phase selection capability.
One separation was done with a UHPLC column with a C18 bonded phase and the other with a UHPLC column with a C18-PFP bonded phase.
The additional separation mechanism provided by the C18-PFP bonded phase makes the overall separation better.
Figure 3: ACE Excel for superior resolution and peak shape: UHPLC results for drugs and their related substances

Conditional column size: 50 x 2.1mm
Mobile phase:
A = 5 mM formic acid (dissolved in water)
B = 5 mM formic acid (dissolved in methanol)
Gradient: from 3 to 100% B in 5 minutes
Flow rate: 0.6 mL/min
Temperature: 40 °C
Detection: UV 254 nm
sample
Paracetamol
2. Hydrochlorothiazide
3. Methylphenyl sulfoxide
4. Methyl sulfone
Chromatograms were generated equally rapidly on C18 UHPLC columns and C18-PFP columns.
However, C18-PFP columns provide better selectivity for peak pair (13, 14) and (15, 17), thus providing superior overall separation performance.
2. The ACE and ACE Excel silica-based matrix stationary phases virtually eliminate the adverse effects of silanol groups on chromatographic separation and have earned a reputation for providing superior peak shapes for basic compounds.
Peak tailing reduces resolution, reduces sensitivity, and even interferes with accuracy and precision (Figure 4).
There are many potential causes for peak tailing, but the main reason for the separation of basic materials is the interaction between the acidic silanol groups on the surface of the silica stationary phase support particles and the amino groups on the analyte (Figure 5).
Figure 4: Effect of peak tailing on resolution and sensitivity

As the peak tail (Tf, tailing factor) increased from 1.0 to 2.0, the resolution (Rs) decreased from 1.5 to 0.87.
As the peak width increases and the peak area remains the same, the sensitivity (peak height) also decreases as the peak tail increases.
The ACE column is made of ultra-inert silica with very low silanol activity.
This ultra-pure silica gel uses patented technology for effective bonding and complete sealing.
The stationary phase leading to this silica matrix virtually eliminates the adverse effects of silanol groups on chromatographic separation.
The ultra-inert nature of the ACE column makes it ideal for the separation of polar basic compounds.
When separating complex basic compounds, ACE columns always give significantly better peak shape and efficiency than other modern alkaline deactivation columns (Figure 6).
Figure 5: Peak tailing interaction

The acidic silanol groups on the surface of the silica stationary phase support form an ion exchange site that interacts with the basic compound.
This ion exchange interaction typically results in peak retention (secondary retention) and causes peak tailing when separating amine compounds using reverse phase HPLC.
 A silica-based stationary phase that almost eliminates the adverse effects of silanol groups on chromatographic separation
A silica-based stationary phase that almost eliminates the adverse effects of silanol groups on chromatographic separation Figure 6: Peak tailing comparison condition Column size: 50 x 2.1 mm
Mobile phase: 40% methanol, 60% water flow rate: 0.2 mL/min
Temperature: 22oC
Sample: pyridine

The number of plates of the basic compound (pyridine) on various common C18 columns is reported.
The number of plates was measured at 10% peak height to allow peak tailing to be included in the measurement.
ACE C18-PFP achieves the highest number of plates due to its ultra-inert nature.
3. ACE Excel brings the renowned ACE HPLC performance to UHPLC <br> It is important to recognize that column selectivity and efficiency are independent variables, you don't have to choose one when developing a separation method. Use another one. In fact, in order to achieve the best overall separation, you should optimize both variables and retention values. This is especially true when high speed separation is required. (See Figure 7)
Figure 7: Using a column efficiency and bonded phase selectivity to develop a faster separation method

condition:
Mobile phase:
A = 5 mM formic acid (0.189) ml / L;
A = 5 mM formic acid (dissolved in methanol)
Column temperature: 40 ° C
Detection: PDA, 254nm
sample:
Aspirin
2. phenacetin
3. 1,3-dinitrobenzene
4. Ethyl benzoate
5. Nimesulide
6. Ibuprofen
7. Indomethacin
With a higher efficiency on UHPLC columns, shorter columns can be used at higher mobile phase flow rates to reduce the separation time of this mixture by 80% (compared to HPLC columns).
However, the separation time can be further shortened by optimizing the selectivity of the bonded phase, in this case utilizing the enhanced selectivity of the C18-PFP bonded phase.
Compared to C18UHPLC columns and C18 HPLC columns, the ACE Excel C18-PFP UHPLC column reduces the separation time of the mixture by more than 80% and 96%, while maintaining excellent resolution of all peaks.
The ACE Excel UHPLC column is designed to take advantage of low-diffusion, ultra-high pressure UPLC® and UHPLC instruments (up to 1000 bar) and is compatible with all commercially available UPLC and UHPLC systems.
ACE Excel UHPLC columns provide better peak shape for basic compounds, improve column-to-column reproducibility, provide additional stationary phases that utilize multiple separation mechanisms, and improve column durability.
The availability of ACE Excel UHPLC columns means that chromatographers have more choices when using UPLC and UHPLC instruments for better results.
Figure 8: ACE Excel columns provide fast, high resolution separation conditions: ACE Excel C18, 2 μm, 3.0 x 50 mm
Mobile phase: from 30% B to 100% B in 60 seconds
A = water + 0.1% TFA B = acetonitrile flow rate: 2.5 mL / min
Column temperature: 40oC
Pressure: 703 bar (10,335 psi)
Detection: PDA, 254 nm
Instrument: Agilent 1290 UHPLC
Compound
Acetanilide
2. Acetophenone
3. Propiophenone
4. Phenylbutanone
5. Benzophenone
6. Phenylpentanone
7. Phenylhexanone
8. Benzheptanone
9. Benzophenone

The ACE Excel C18 column provides baseline separation of these nine analytes in less than 60 seconds.
4. ACE HPLC columns and ACE Excel UHPLC columns have proven to be durable, reliable, and have excellent column life.
The acidified hydrolysis of the siloxane bonded to the silica stationary phase support can lose the bonded phase, thereby reducing the lifetime of the column. The acid hydrolysis increases as the pH of the mobile phase decreases and the column temperature increases.
Shorter alkyl phases such as C4 and C3 and the phenyl phase are generally more susceptible to loss of the bonded phase than the C18 phase. In addition, low purity silica gel stationary phase supports and low surface coverage stationary phase supports make some bonded phases more susceptible to acid hydrolysis.
The ACE bonded phase exhibits extremely high stability due to the use of ultra-high purity silica-immobilized carrier particles and extremely high surface coverage of the bonded phase. (See Figure 9)
Although all ACE bonded phases are excellent in stability, some bonded phases are not as stable as other bonded phases. For example, it is generally believed that the C18 bond is more stable than the alkyl phase, the PFP phase, and the phenyl bonded phase having a shorter column length.
In fact, poor stability of a typical phenyl stationary phase is one of the main reasons why this phase is not often selected in method development.
To solve this problem, ACE C18-AR was developed, which allows chromatographic analysis to utilize the powerful π-π and dipole-dipole separation mechanisms while still enjoying the robustness and durability of the C18 phase.
As shown in Figure 10, ACE C18-AR not only has very excellent stability to a typical phenyl bonded phase, it even exhibits better stability than many other C18 bonded phases.
Similarly, ACE C18-PFP provides a variety of separation mechanisms that can be used to achieve better overall separation while benefiting from the stability of the C18 phase.
Figure 9: Acid stability, pH 1.8
Conditional column: ACE 4.6 x 150 mm
Mobile phase: 50% CH3CN, 50% water flow rate: 1.0 mL/min
Temperature: 22 °C
Analyte: phenanthrene acid exposure conditions:
Mobile phase: 50% CH3CN 50% 0.1% TFA (dissolved in water) (pH 1.8)
Flow rate: 1.0 mL/min
Temperature: 22 °C

Figure 10: Acceleration column stability study: 80 ° C at pH 1.9
Acidic exposure conditions:
Mobile phase: 5:95 MeOH/0.1% TFA (dissolved in water) (pH 1.9)
Flow rate: 0.20 mL/min
Temperature: 80 °C
Column size: 50 x 2.1mm
Analyte: Philippine

Using conditions designed to accelerate column degradation, the ACE C18-AR phase exhibits minimal retention losses and its lifetime corresponds to the highly stable ACE C18 phase. Both phases are made of the same ultrapure silica and are more durable than Zorbax SB-C18 (Zorbax SB-C18 was once considered a stationary phase with excellent stability at high temperatures and low pH) .
As expected, C18 bonded columns based on low purity silica gel (Waters Spherisorb ODS2) have a much reduced lifetime under these accelerated conditions.
Of particular note is the comparison of the lifetime of conventional phenyl columns with ACE C18-AR. Compared to ACE C18-AR, the lifetime of phenyl columns is reduced despite the use of high purity silica, suggesting that ACE C18-AR may be suitable for applications where phenylpropyl columns have a short lifetime.
It is quite common to attach HPLC and UHPLC to mass spectrometers commonly referred to as LC-MS.
However, mass spectrometry is very sensitive to traces of the bound phase (called column "bleed") that may elute from the HPLC/UHPLC column. Column bleed may interfere with the analytical results and, importantly, the column selected in the LC-MS application has a sufficiently stable bonded phase to avoid excessive column bleed.
Figure 11 shows that the ACE C18-PFP column has little "loss" interference with LC-MS analysis.
The stability of C18-PFP (and ACE C18 and ACEC18-AR) makes these columns more suitable for LC-MS applications.
Figure 11: Low column bleed of the ACE C18-PFP column makes it an ideal choice for LC/MS applications. Column size: 50 x 3.0 mm
Mobile phase: Gradient: 5 to 95% B in 5 minutes, 5 minutes in 95% B
A: 0.1% formic acid (dissolved in water)
B: 0.1% formic acid (dissolved in acetonitrile)
Flow rate: 0.43 mL/min
Temperature: 60oC
Detection: Agilent 1100 MSD ESI positive ion fast scan mode, fast scan all 50 – 1000 m/z
Sample: blank run

Column bleed can interfere with the detection and measurement of target analytes in LC-MS applications.
Column loss was not shown on the ACE C18-PFP column.
The low bleed characteristics of all ACE columns make them ideal for LC-MS applications.
UHPLC columns may lack durability. Coupled with the possibility of blockage of the inlet screen, you may face column durability that is not as problematic as an HPLC column.
The ACE Excel column is very tolerant and offers the same durability and longevity you expect from an HPLC column.
ACE Excel will change your view of the durability of UHPLC columns.
The ACE Excel column is not only better packed, but also features a patented inlet screen design that makes it less susceptible to blockage than other UHPLC columns.
It is still recommended that you filter the sample and mobile phase as you would any UHPLC column, but the ACE Excel column is more tolerant than other UHPLC columns and offers the same durability and longevity as HPLC columns.
Figure 12: ACE Excel columns have a good reputation for durability

A 2.1 x 100 mm ACE Excel C18 UHPLC column was run over 2000 gradients at an average pressure of 1,000 bar (14,500 psi).
At the end of the stability study, the efficiency (plate number) and retention time remained essentially unchanged.
5. ACE Excel columns have excellent reproducibility.
The reproducibility between the column and the column is affected by the formation of the silica stationary phase support, the binding of the stationary phase to the stationary phase support, and the filling of the stationary phase in the column.
During the preparation of the column, the better each stage is controlled, the better the reproducibility and quality of the column.
The ACE column is fully controlled at every stage of the column preparation process.
These controls ensure that ACE columns produce reliable and predictable performance between columns and between batches.
Figure 13 provides an example of a typical batch verification test.
Subtle changes in silanol activity are one of the main reasons for the selective change between the column and the column.
ACE and ACE Excel columns offer excellent reproducibility due to the use of ultra-pure reagents and rigorous manufacturing process control (high purity silica stationary phase media with uniform surface characteristics).
Combining this high-purity silica with advanced bonding technology produces a range of highly inert stationary phases that virtually eliminate silanol interference in the chromatogram, providing superior column-to-column weight Present.
 |  |
6. ACE columns provide easy scalability from UHPLC to HPLC to preparative HPLC.
The same quality control ensures high reproducibility of different batches, and also makes it easier to convert between UHPLC and HPLC methods, or to scale up to preparation applications when separating and purifying target compounds.
The efficiency of the column will of course change, but it is expected that the band spacing (selectivity) will be the same.
Figure 14 illustrates that when the same ACE bonded phase but different particle size packed columns are used for operation, the analyte-consistent band spacing type (selectivity) can be foreseen.
Figure 14: Selectivity retention and assurance when the ACE stationary phase was extended from UHPLC (2 μm) to HPLC (3 μm and 5 μm) to preparative HPLC (10 μm).
Conditional mobile phase: 35:65 MeCN/0.1% TFA (dissolved in water)
Temperature: 22 °C
Wavelength: 254 nm
Analyte
Uracil
2. 4-hydroxybenzoic acid
3. Acetylsalicylic acid
4. Benzoic acid
5. 2-hydroxybenzoic acid
6. Ethyl p-hydroxybenzoate

These chromatograms of the test samples were run on the same column with the same bonded phase but different particle sizes, and the results elucidated the ease with which the separation was extended from UHPLC to HPLC to preparative HPLC.
When using ACE Excel UHPLC columns for rapid method development, and the method must be transferable to HPLC, ease of scalability is particularly valuable.
Figure 15: ACE stationary phases provide the same selectivity, whether they are UHPLC, HPLC or preparative HPLC column conditions: 2.1 x 50 mm
Mobile phase: from 30% B to 90% in 1.4 minutes B
A = 20 mM ammonium formate + 0.1% formic acid
B = acetonitrile + 0.1% formic acid gradient: 0.30 minutes at 30% B, 30 to 90% B in 1.10 minutes, 0.40 minutes at 90% B Flow rate: 0.5 mL/min
Column temperature: 30oC
Detection: AB Sciex Triple Quad(TM) 5500 MS, operating in ESI (+) mode: Shimadzu Prominence UFLC System Analyte
Mebendazole
Proprolin
3. Amitriptyline
4. Loperamide

These chromatograms of the test samples were run on the ACE Excel C18 2 μm U HPLC column, ACE C18 3 μm HPLC column, and ACE C 185 μm column with the same mobile phase conditions. The results demonstrate that the selectivity of the ACE stationary phase is consistent regardless of the particle size of the fill.
This makes the transfer of methods from UHPLC to HPLC or HPLC to UHPLC easier. It also makes it easier to extend from analytical applications to preparative applications.
Disposable Latex Gloves,Disposable Vinyl Gloves,Nitrile Glove,Latex Glove
ZHANGJIAGANG DINSHENGLIN TRADING CO.,LTD , https://www.dslhouse.com