The results of the second phase of the new drug for cardiomyopathy are all positive, and will enter phase 3
March 12, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];MyoKardia, a biopharmaceutical company that develops the treatment of hereditary cardiovascular diseases, recently announced the full positive results of the new drug mavacamten in the phase 2 clinical study PIONEER-HCM in symptomatic, obstructive hypertrophic cardiomyopathy (oHCM). . The results of this time included data from the low dose patient cohort (Cohort B).

According to the American Heart Association (AHA), hypertrophic cardiomyopathy is a very common cardiovascular disease and a common cause of cardiac arrest in young people. Most patients do not have any symptoms in their daily lives. However, a small number of patients may have symptoms such as myocardial obstruction, and their disease is also called symptomatic, obstructive hypertrophic cardiomyopathy. Although this part of the patient is rare, it suffers from great health risks and requires new drugs for treatment.
Mavacamten is an innovative oral myocardial myosin allosteric modulator that is expected to reduce the symptoms of excessive contraction in these patients. In April 2016, the FDA issued orphan drug qualifications to mavacamten.
In a Phase 2 clinical study called PIONEER-HCM, the researchers evaluated the safety, tolerability, pharmacokinetics, and efficacy of mavacamten. The test consisted of two dose groups. The first group of subjects received mavacamten and discontinued other beta-blocker drugs. The second group of subjects will receive a low dose of mavacamten and will not need to stop beta-blocker therapy. During the 12-week treatment period of the first group, all 10 subjects achieved an average LVOT (left ventricular outflow tract) pressure gradient peak from baseline reduced by 125 mmHg. This also provides a basis for the second group of low-dose studies.
The primary goal of PIONEER-HCM Cohort B was to evaluate the efficacy and safety of lower doses of mavacamten (2 mg and 5 mg) in patients with oHCM. Both cohorts of PIONEER-HCM reached the primary endpoint, reducing post-exercise LVOT gradient from baseline to week 12 and were statistically significant (cohort A p=0.002, cohort B p=0.020). Specifically, the LVOT gradient after exercise was observed to decrease from the baseline 86 mmHg mean to 64 mmHg at week 12. In addition, the resting LVOT gradient decreased from 86 mmHg at baseline to 38 mmHg at week 12 (p = 0.004). Left ventricular ejection fraction (LVEF) at rest was relatively small from baseline, and patients in cohort B maintained an ejection fraction above 50%. Several key secondary endpoints, including the New York Heart Association (NYHA) classification and improvement in dyspnea scores, also reached statistical significance in each cohort. Cohort A showed statistical significance at the secondary endpoint of changes in exercise capacity (VO2 peak), and cohort B showed a positive trend of improvement. In summary, data from the PIONEER-HCM study indicate that the optimal daily dose for most patients is between 5 and 15 mg. These data are the three key clinical trials of the MyoKardia program, EXPLORER-HCM, which provides initial dose and guided dose adjustment using mavacamten in symptomatic oHCM patients. EXPLORER-HCM is expected to begin in the second quarter of 2018.
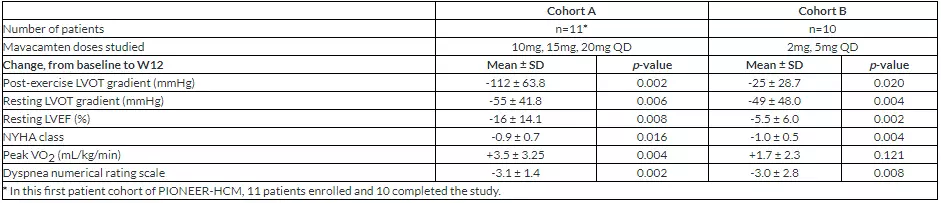
â–²The data of this clinical trial (Source: MyoKardia official website)
"The life of symptomatic oHCM patients is limited by the deterioration of their condition. Unfortunately, current treatment options are either invasive or limited in efficacy." Daniel Jacoby, Professor, Yale University School of Medicine, Principal Investigator, PIONEER-HCM Research “In the Phase 2 PIONEER-HCM study, we found that patients taking mavacamten feel better in terms of eliminating LVOT obstruction, increasing function and exercise capacity, and quantifying the quantitative and qualitative measurements of shortness of breath. Inspired, I look forward to mavacamten entering the Phase 3 EXPLORER study."
“The data from the two cohorts of PIONEER-HCM studies gave us a better understanding of the relationship between mavacamten's dose and pharmacodynamic effects in treating oHCM patients,†said Dr. Marc Semigran, Chief Medical Officer of MyoKardia. We entered the key Phase 3 EXPLORER-HCM trial and we believe we can optimize the treatment of mavacamten to improve the patient's symptoms and function while maintaining the left ventricular ejection fraction."
We expect this new drug to go smoothly in Phase 3 clinical trials to treat more patients with this type of heart disease.
Reference materials:
[1] MyoKardia Sees Positive Results in Mid-Stage Heart Trial
[2] MyoKardia Announces Positive Results from Low-Dose Cohort of Phase 2 PIONEER-HCM Study of Mavacamten in Symptomatic, Obstructive Hypertrophic Cardiomyopathy Patients
[3] WuXi PharmaTech - a new drug for hypertrophic cardiomyopathy mavacamten clinical phase 2 trial results positive
Fireproof Safe is a good choice. It can not only provide a safe storage space for these articles, but also protect these articles from loss in the event of unfortunate fire. Steel anti-theft safety fireproof safe adopts special sealing technology and fire prevention materials, which can prevent fire source from entering its interior and maintain the integrity and safety of articles. In addition to fire prevention, electronic fireproof safe can also effectively prevent damage to water, dust and other articles. Our fireproof safe has a fire test report, which can effectively prevent external heat and smoke and prevent internal articles from being damaged in the fire test. The Paper documents in the box are not damaged within one hour of internal melting point 177 ℃. Our factory has more than 40 years of production experience, and we can OEM or ODM for you. Now we are looking for more partners worldwide. Sincerely hope to get your attention and cooperation opportunities.
Electronic Fireproof Safe,Fingerprint Fireproof Safe,Home Digital Code Fireproof Safe box,Office Fingerprint Fireproof Safe box,Steel Anti-theft Safety fireproof safe
Hebei Hupai Aodi Cabinet Industry Co.,Ltd. , https://www.hupaiaodi.com