The latest breakthrough in stem cell therapy: human "chimeric cells" restore DMD key proteins
March 19, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, a new study from scientists at the University of Illinois at Chicago (UIC) showed that "chimeric cells" are obtained by fusing normal human muscle cells with muscle cells of patients with Duchenne muscular dystrophy (DMD). ), implanted in the muscles of DMD model mice, can significantly improve the muscle function of DMD model mice.
This latest research has been published online today in the International Stem Cell Review and Reports, entitled: Dystrophin Expressing Chimeric (DEC) Human Cells Provide a Potential Therapy for Duchenne Muscular Dystrophy [Expression Resistance Mutant (DEC) human cells of dystrophin provide a potential therapy for DMD]
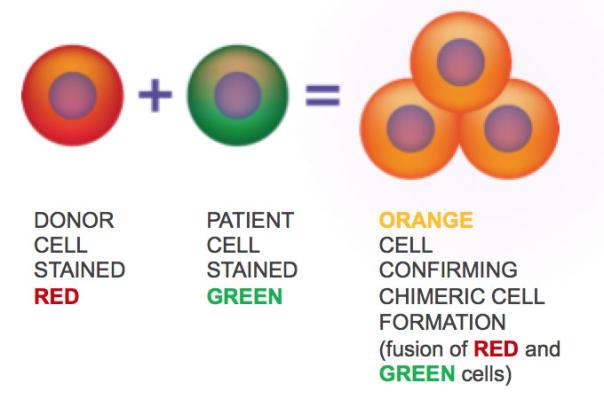
Fusion of donor cells (red) with patient cells (green) to prepare chimeric cells (orange)
These "chimeric cells" are obtained by passing a cell of a normal donor (donor) containing a functional dystrophin gene with a recipient from a DMD (recipient). The cells are prepared by fusion. Anti-dystrophin is a structural muscle protein that is absent or dysfunctional in DMD patients. In another study published in the same journal in January 2018, researchers used chimeric cells from normal mouse donor cells and DMD model mouse recipient cells to implant these chimeric cells into a small DMD model. After the muscles of the rats, it was found that the level of dystrophin was increased by 37%, and muscle function was improved. These new cells are both characterized by donors and receptors, interact with the surrounding environment like normal cells, and survive and express dystrophin for up to 30 days.
The study was conducted by a research team led by Dr. Maria Siemionow, professor of plastic surgery at UIC Medical School. Now, the team used the chimeric cells prepared by human cells to implant DMD model mice and obtained similar results.
Professor Siemionow said the findings lay the foundation for the use of long-lived chimeric cells as a novel DMD therapy and look forward to clinical trials in humans in the near future.
DMD is an X-linked genetic disease characterized by progressive muscle degeneration and weakness, primarily affecting men. Worldwide, one in about 4,000 newborn boys is affected by the disease. DMD patients develop symptoms during early childhood, usually between the ages of 3-5. The disease can lead to muscle weakness and loss of motor function, ultimately leading to respiratory failure or heart failure and death. With the advancement of treatment, many DMD patients have lived in their 20s and some have lived in their 30s, but there is no cure for this disease. DMD is caused by mutations in the dystrophin gene, leading to dystrophin loss or dysfunction, a structural protein that plays a key role in the function of muscle fibers.
Currently, more promising DMD treatments include gene therapy and stem cell therapy, but both have their own shortcomings. Gene therapy relies on the delivery of a gene from a normal version of a deleted or dysfunctional gene to a cell by a virus. The disadvantage of this method is that not only does the cell have an immune response to the viral infection, but the treatment is not effective, and there is no guarantee that the virus will only be infected. cell. Stem cell therapy is performed by implanting stem cells containing normal genes into the patient, but this method has the disadvantage of requiring the patient to take an immunosuppressive agent to prevent rejection.
Professor Siemionow said that the chimeric cell therapy developed by the team is not a traditional stem cell therapy. Chimeric cell therapy does not require the patient to take anti-rejection drugs to gradually restore dystrophin levels because these implanted cells can escape the immune system of the implanted person. In traditional stem cell therapy, the implanted cells are 100% other individuals, and the implanted need to receive anti-rejection drugs to prevent the body's immune system from destroying these foreign cells. In contrast, 50% of chimeric cells are autologous, have many biochemical and genetic characteristics of the implanted, and thus can deceive the immune system to ignore them, in other words, the chimeric cells and the implanted themselves. The cells are very similar, and the body's immune system sends a "pass" to these chimeric cells.
Dr. Kris Siemionow, co-author of the paper and associate professor of plastic surgery at UIC Medical School, said that if such chimeric cells are used to treat patients with DMD, they can use the normal muscle cells from the patient's father or close relatives to fuse with the patient's own muscle cells. . In the lab, the researchers fused muscle cells from normal healthy donors to muscle cells from DMD patients and found that the resulting chimeric cells expressed dystrophin. When these cells were implanted into the leg muscles of DMD model mice, the level of dystrophin in the muscle fibers affected by the transplanted chimeric cells increased by about 20% 90 days after implantation, which is sufficient Significant improvement in muscle function. Specifically, tests were performed on implanted mice and found to have more than 60% improvement in muscle function tests and more than 20% improvement in muscle fatigue tolerance tests.

Professor Maria Siemionow and Associate Professor Kris Siemionow
It is worth mentioning that Maria Siemionow and Kris Siemionow are mother-child relationships. Just earlier this year, they founded Dystrogen Therapeutics Pharmaceuticals Inc. to develop its innovative chimeric cell therapy into a DMD therapy. (Sina Pharmaceutical Compilation/newborn)
Article reference source:
1. Human 'Chimeric' Cells Restore Crucial Protein in Duchenne Muscular Dystrophy
2, Dystrogen Therapeutics company's official website
Monkfish-Sliced,Monkfish-Fillets,Monkfish Tails With Skinless,Monkfish Tails With Skin-On
GOLD STAR FISHERY ZHOUSHAN CO.,LTD. , https://www.goldstar-aquatic.com