Release date: 2016-06-06

Lung cancer is one of the deadliest tumors in the world. In China, the incidence and mortality of lung cancer rank first in all malignancies. In 2012, there were approximately 653,000 new lung cancer patients in China, and about 597,000 deaths. In the United States, according to NIH data, 221,200 people were diagnosed with lung cancer each year, and the death toll was 158,000. Non-small cell lung cancer (NSCLC) is the predominant type of lung cancer, accounting for 80% to 85% of all lung cancers, 10-20% for EGFR exon 19 deletion and exon 21 L858R variation.
Since tumor DNA from patients with non-small cell lung cancer is involved in the patient's blood, it is possible to detect specific mutations in the patient's blood. This method of detecting tumor DNA in a blood sample is referred to as a "liquid biopsy."
EGFR–TKIs targeting drugs, the preferred treatment for patients with EGFR gene mutations
Targeted drugs such as epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are the preferred treatment for patients with EGFR gene mutations. In patients with advanced non-small cell lung cancer with EGFR mutations, studies have demonstrated that EGFR-targeted therapy can significantly reduce the risk of disease progression or death and improve the quality of life of patients.
Roche's EGFR inhibitor Tarceva ® (TAGRISSOTM) was approved for second-line therapy for non-small cell lung cancer in 2004 and was approved as a first-line therapy for patients with EGFR mutations in 2013.
FDA approves the first liquid biopsy technology, Roche EGFR mutation detection "winning"

On June 1, 2016, the US FDA approved the first "liquid biopsy" method based on EGFR gene mutation - Roche cobas ® EGFR Mutation Test v2 for the detection of EGFR exon 19 deletion and non-small cell lung cancer (NSCLC) patients. The L858R substitution mutation of Receptor 21.
It is worth mentioning that the cobas ® EGFR Mutation Test v2 test has been marketed in many European countries (CE-IVD certified) and can detect 42 variants, including EGFR-resistant T790m variants.
In February 2015, China's CFDA updated the Tarceva ® instructions for the approved non-small cell lung cancer treatment. Based on the EGFR gene mutation test, if the tumor specimen is not evaluable, it can be obtained from blood (plasma) specimens. ctDNA was evaluated.
Dr. Alberto Gutierrez, Director of the Department of In Vitro Diagnostic and Radiological Health at the FDA Center for Devices and Radiation Health, said that the approval of “liquid biopsy†has pushed medicine toward “precision†and that “liquid biopsy†will become a “least invasive†way. To help doctors diagnose specific mutations in cancer patients. This FDA approval provides a means of choice for treatment in critically ill patients or patients who are unable to perform a biopsy for other reasons.
Three methods of detection for "liquid biopsy"
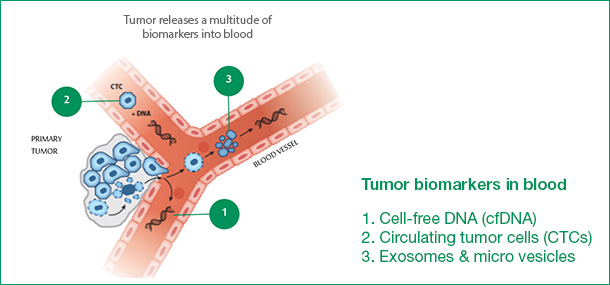
"Liquid biopsy" includes three detection methods, followed by detection of free DNA (cfDNA), detection of circulating tumor cells (CTCs), detection of extracellular bodies (see below); cobas ® EGFR Mutation Test v2 is detection Free DNA (cfDNA).

For a variety of reasons, the detection rate of lung cancer tissue with EGFR gene mutation in clinical practice is not high, which makes it difficult to implement individualized treatment strategies based on driving gene mutation targets. A number of retrospective and prospective studies have shown that blood free DNA (cfDNA) or circulating tumor cells (CTCs) are a suitable alternative to mutation analysis when tumor tissue is difficult to obtain.
Advantages and disadvantages of "liquid biopsy"
Compared with traditional tumor tissue biopsy, the “liquid biopsy†of blood extraction requires no complicated operation, so the cost is greatly reduced; and the invasiveness is low, which greatly increases the convenience of use and is beneficial to improve the detection rate of EGFR gene mutation in clinical practice; In addition, EGFR gene mutation detection in ctDNA is highly specific (100%, 99.8%, and 97.2% specificity in IPASS, IFUM, and IGNITE studies, respectively).
Due to the heterogeneity of tumor tissue, the sampling of in vivo tests may not be representative; and the sensitivity is relatively low (43.1%, 65.7%, and 49.6%, respectively), which may be related to tumor stage, blood sample processing, and detection methods. .
Therefore, if the blood EGFR gene mutation test results are negative, a tumor tissue biopsy is needed to determine whether there is a mutation in the EGFR gene.
Market outlook and challenges of “liquid biopsyâ€
According to BCC analysis, the market value of “liquid biopsy†will reach US$22 billion in 2020, and China also predicts a market of RMB20 billion. Jay Flatley, CEO of sequencing giant Illumina, said in an interview with the media that the market for liquid biopsy is at least $40 billion, and even claims that the technology may be the most exciting breakthrough in cancer diagnosis.
Since the nucleic acid tissue involved in "liquid biopsy" is extremely low in peripheral blood and body fluids, accurate detection is technically an obstacle, but precision medicine is a general trend in the treatment of the entire disease, especially tumor treatment.
More convenient and cheaper biomarker detection methods are crucial for patient selection in precision medications and clinical trials, especially in the current situation where new drug prices and new drug development costs are close to the limit.
Source: Bio-Exploration
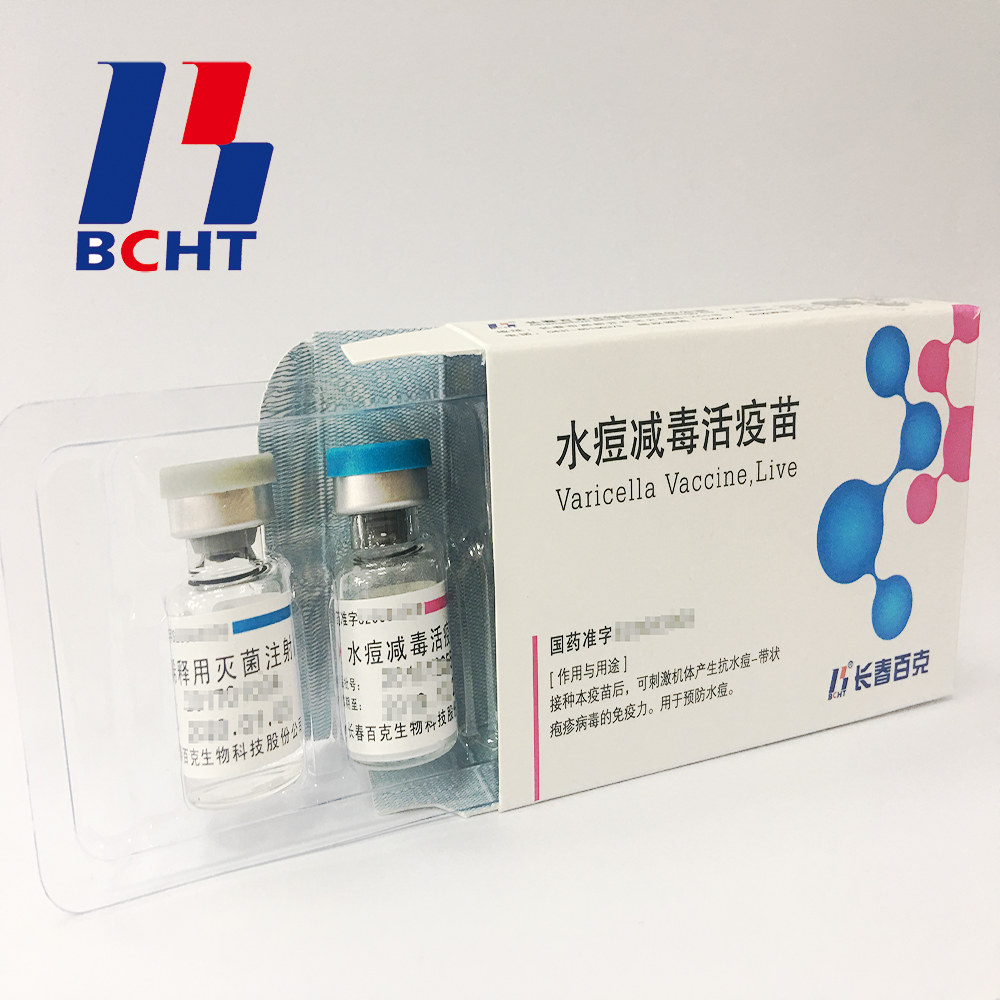
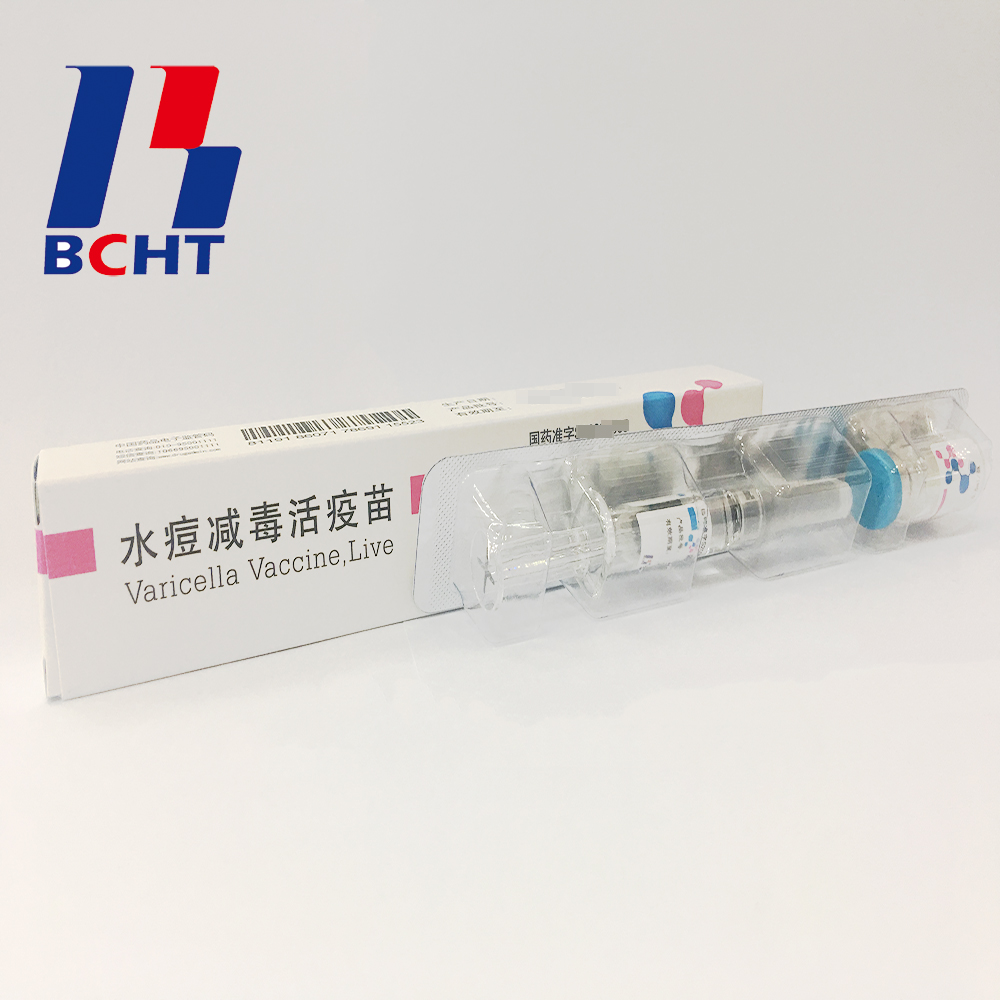
Varicella Vaccine Finished Products
Finished products of varicella vaccine. It has three qualities,good safety of gelatin-free, long validity period by good stability, better protection with high titer and immune efficacy. These improvements enhanced the vaccine safety and quality, and established BCHT the leading position in varicella vaccine. We have two different packages, penicillin bottle and pre-filled syringe. And it has been exported to other countries, such as India, Philippines.
Finished Products Of Rabies Vaccine,Rabies Vaccine For Human Use,Live Biotechnology Chicken Pox,Live Lyophilized Vaccination
Changchun BCHT Biotechnology Co. , https://www.ccbcht.net