Summary of recent progress in the field of diabetes research (04.19)
April 19, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. FDA approves BYDUREON® injection suspension as an additional treatment for basal insulin
Recently, AstraZeneca announced that the US FDA approved BYDUREON® (exenatide extended release) injection suspension as an additional treatment for basal insulin for adult patients with type 2 diabetes who have inadequate glycemic control. BYDUREON has been approved for adult patients with type 2 diabetes who have poor glycemic control. These patients are still taking one or more hypoglycemic agents in addition to diet and exercise.

Diabetes is a growing global pandemic, and as of 2015, 400 million adults worldwide were affected by diabetes. People with type 2 diabetes are resistant to insulin or are unable to secrete enough insulin to maintain normal blood sugar levels. Sustained high blood sugar levels can lead to complications of diabetes such as heart disease, stroke, kidney failure, neuropathy, lower extremity amputation and blindness. Therefore, controlling blood sugar is a key part of diabetes treatment.
BYDUREON is a weekly GLP-1 receptor agonist injection for adult patients with type 2 diabetes. It helps the body produce more insulin in response to increased glucose, reduces glucagon production, and slows the stomach tract. Empty, thus reducing high blood sugar. BYDUREON was first approved by the FDA in January 2012. Both the American Diabetes Association and the American Association of Clinical Endocrinologists support the use of GLP-1 receptor agonists in combination with basal insulin and metformin to help control type 2 diabetes patients with blood sugar.
▲ BYDUREON® (exenatide extended release) injection suspension (Source: BYDUREON official website)
The approval was based on the results of a 28-week study of DURATION-7. The study evaluated the efficacy of BYDUREON or placebo as an additional treatment for insulin glargine in adult T2DM patients with or without metformin. The results showed that in patients with an average baseline HbA1c of 8.5%, the mean HbA1c was reduced by 0.9% in the BYDUREON group (n=231), while the placebo group (n=229) was only reduced by 0.2% (the difference between groups was 0.6%). , p < 0.001). In addition, 32.5% of patients in the BYDUREON group had HbA1c <7.0%, compared with 7.0% of patients in the placebo group.
No new security issues were identified in the study. Overall hypoglycemia was similar in the two groups (29.7% in the BYDUREON group and 29.0% in the placebo group) with no severe hypoglycemia. Like other GLP-1 receptor agonists, when BYDUREON is used with insulin, it increases the risk of hypoglycemia, and doctors should consider lowering the dose of insulin.
Dr. Jim McDermott, Vice President, Medical Affairs, AstraZeneca Diabetes, said: "Type 2 diabetes is a complex disease for patients and health care providers. This is why we continue to invest in scientific advances to support the safety of exenatide. For reasons of sexuality and efficacy, even the first exenatide formulation has been on the market for 13 years. DURATION-7 is part of a broader DURATION clinical trial project that continues to generate important insights into the use of exenatide. BYDUREON is one of the most widely studied clinical trials of GLP-1 receptor agonists and has been studied in more than 19,000 patients. With this approval, we have brought another important benefit to healthcare providers. Treatment options to treat patients with type 2 diabetes who are using basal insulin but have inadequate glycemic control."
2 , the first new drug based on beta cell-based diabetes submitted for clinical application
Recently, ARKAY Therapeutics announced that it has submitted a new drug clinical trial (IND) application for RK-01 to the US FDA. RK-01 is a "first-in-class" "beta-cell-centric" drug combination for newly diagnosed and obese patients with type 2 diabetes. The drug contains three components, namely metformin (metformin), valsartan (valsartan) and celecoxib (celecoxib). For the problem of insufficient glycemic control in patients with type 2 diabetes, RK-01 will conduct the first human proof of concept test.

Type 2 diabetes is a global epidemic, which occurs after 35-40 years of age, accounting for more than 90% of diabetic patients, affecting more than 400 million people worldwide. Diabetes is the leading cause of blindness, kidney failure, heart attack, stroke and lower extremity amputation. In 2015, there were 1.6 million deaths directly caused by diabetes. Current treatments primarily control symptoms, but do not alleviate potentially complex causes. There is still huge unmet medical needs in this area.
The unique formulation of RK-01 adds valsartan and celecoxib to metformin, which blocks several complementary mechanisms of inflammation and hyperglycemia, which lead to a gradual deterioration of islet beta cell function, which is exactly 2 The core of complex pathophysiology of type 2 diabetes. Valsartan is an angiotensin II receptor type 1 blocker that has been approved for the treatment of hypertension; celecoxib is a selective cyclooxygenase-2 (Cox-2) inhibitor It has been approved for the treatment of arthritis. Activation of the renin-angiotensin system (RAS) and Cox-2 in beta cells is associated with the complex pathophysiology of type 2 diabetes. Cox-2 derived pro-inflammatory prostaglandin PGE2 inhibits GLP-1 agonist-mediated glucose-dependent insulin secretion (GDIS) from beta cells. Currently, some commercially available drugs are GLP-1 agonists, but the existing treatments cannot block the damage of β-cell function caused by inflammation. While RK-01 can maintain or restore beta cells, it provides long-lasting glycemic control and even delays and prevents the use of insulin.
“We are pleased that ARKAY has submitted a custom combination of IND to fill the most important clinical gap by targeting multiple pathways leading to beta cell dysfunction, a 'core defect' in all patients effectively managing diabetes,†the famous endocrine Dr. Stan Schwartz, MD, and medical consultant of the ARKAY Scientific Advisory Board, said: "Every ingredient in RK-01 has a unique set of properties to maintain or restore beta cell function, improve blood glucose parameters and treat comorbid conditions such as NASH and joints. Inflammation. As we have recently discussed the discussion of diabetes and its complications in a publication on unified pathophysiology, RK-01 may also reduce the risk of diabetic complications in these patients."
3. The first medical device that uses artificial intelligence to screen for diabetes-related eye diseases has been approved by the FDA !
Recently, the US FDA approved the first medical device IDx-DR to use artificial intelligence to detect retinopathy of diabetic patients. IDx-DR is the first medical device to receive marketing authorization to provide screening decisions without the need for a clinician to interpret images or results, which allows doctors who are not normally involved in eye care to use the device.

Diabetic retinopathy occurs when hyperglycemia causes damage to the retinal blood vessels, the photosensitive tissue at the back of the eye. Diabetic retinopathy is the most common cause of blindness in patients and is the leading cause of visual impairment and blindness in adults at working age.
Developed by IDx, IDx-DR is a software program that uses artificial intelligence algorithms to analyze eye images taken by the Recone Camera Topcon NW400. The doctor uploads the patient's digital image of the retina to the cloud server on which the IDx-DR software is installed. If the image quality is acceptable, the software can provide the doctor with one of two results: 1) "Discovering mildly above diabetic retinopathy: referral to an ophthalmologist"; or 2) "No mild to diabetic retinopathy found , review within 12 months." If the test results are positive, the patient should contact an ophthalmologist as soon as possible for further diagnostic evaluation and treatment.
The approval was based on FDA's assessment of clinical study data on retinal images of 900 diabetic patients at 10 major treatment sites. The study was designed to assess the accuracy of IDx-DR in detecting mild to diabetic retinopathy. In this study, IDx-DR was able to correctly identify 87.4% of patients with mild or above diabetic retinopathy, and 89.5% of patients were able to correctly identify no more than mild diabetic retinopathy.
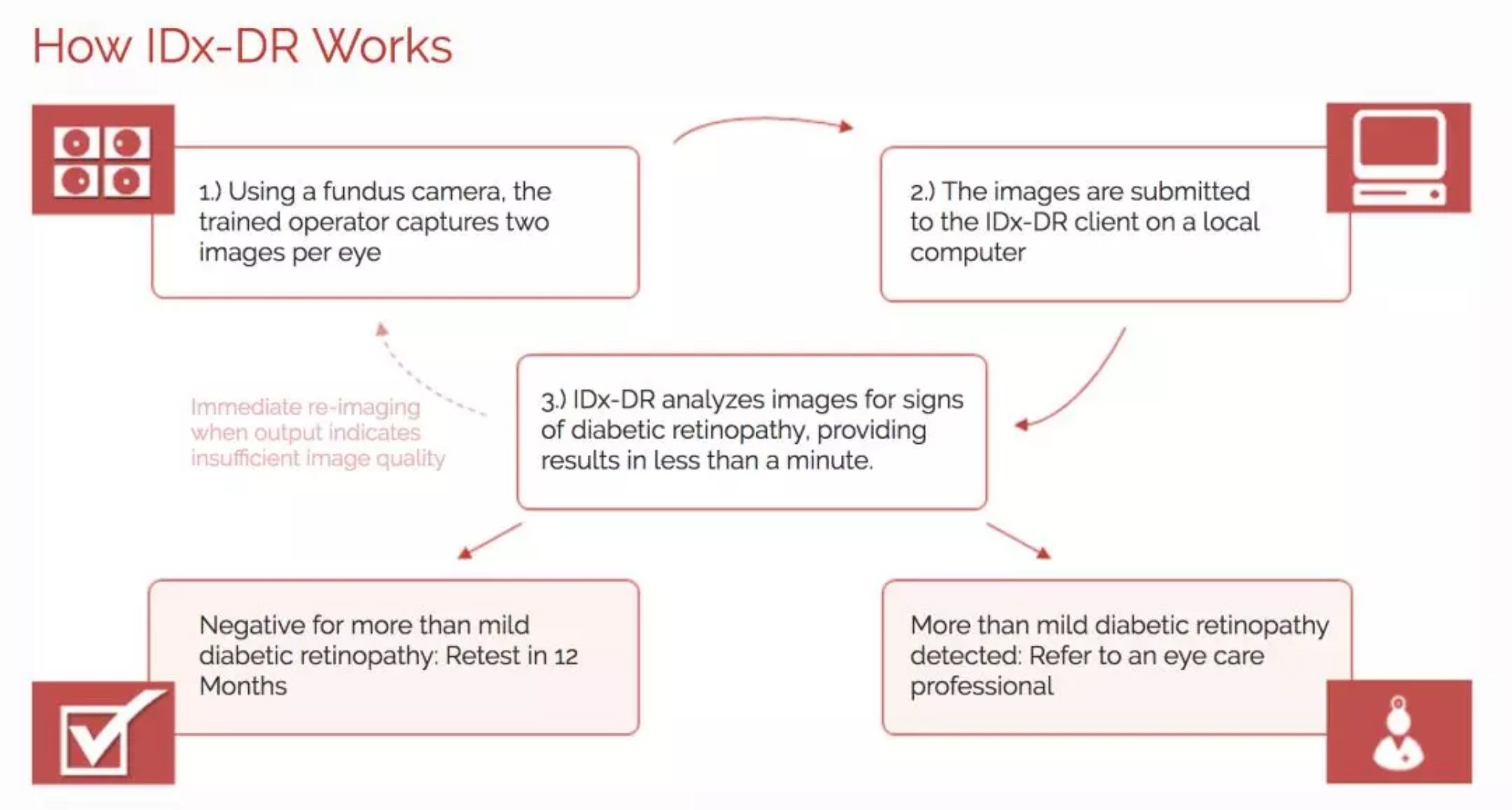
â–² IDx-DR workflow (Source: IDx official website)
Patients not eligible for IDx-DR screening for diabetic retinopathy include history of laser treatment, history of surgery or intraocular injection, persistent loss of vision, blurred vision, floaters, previous diagnosis of macular edema, severe non-proliferative retina Lesions, proliferative retinopathy, radiation-induced retinopathy, retinal vein occlusion, and pregnant diabetics.
"The early detection of retinopathy is an important part of the disease management of millions of diabetic patients, but many diabetic patients do not adequately screen for diabetic retinopathy, because about 50% of them cannot guarantee an annual ophthalmologist," FDA Dr. Malvina Eydelman, Director of the Department of Otorhinolaryngology and Devices, Radiation Health Center, said: "Today's decision makes the new artificial intelligence technology available to primary care physicians' offices. FDA will continue to promote the availability of safe and effective digital medical devices to improve patient access. The health care opportunities you need."
4 , INVOKANA® significantly improves renal function in diabetic patients
Janssen Pharmaceutical Companies of Johnson & Johnson recently announced additional analysis of the landmark CANVAS project, showing that INVOKANA® (canagliflozin) improves renal outcomes in patients with type 2 diabetes with high-risk cardiovascular disease. The data was presented at the 2018 National Kidney Foundation Spring Clinical Conference in Austin, Texas.

Diabetes is a growing global epidemic. As of 2015, 400 million adults worldwide are affected by diabetes. Diabetic nephropathy is an important complication of diabetes and has become one of the most common causes of end-stage renal disease. Due to its complex metabolic disorders, once it reaches the end stage, it is often more difficult to treat than other kidney diseases. Therefore, timely prevention and treatment is of great significance for delaying diabetic nephropathy.
Canagliflozin is a daily oral diabetes drug that belongs to the class of sodium glucose cotransporter 2 (SGLT2) inhibitors. These drugs reduce the body's blood sugar levels by blocking the kidney's reabsorption of blood sugar and increasing the excretion of blood sugar in the urine.
The CANVAS project is the longest and most extensive focus on cardiovascular efficacy in all SGLT2 inhibitors to date. It is also the first project to evaluate the efficacy, safety, and persistence of canagliflozin in more than 10,000 patients with type 2 diabetes who have a history of cardiovascular disease or at least two cardiovascular risk factors. In the new analysis, canagliflozin reduced the urinary albumin to creatinine ratio (UACR), a key biomarker for chronic kidney disease. In patients with renal function retention and reduction, glomerular filtration rate (eGFR) was reduced from baseline. 17% and 23% (P heterogeneity = 0.01). At pre-specified composite endpoints (40% reduction in eGFR, end-stage renal disease or nephropathy death), canagliflozin resulted in a 47% reduction in relative risk in patients with renal function retention (HR 0.53, 95% CI 0.39-0.73, P heterogeneity) =0.28), the risk was reduced by 24% in patients with reduced renal function (HR 0.76, 95% CI 0.49-1.17, P heterogeneity = 0.28). Similar results were observed in studies in which creatinine doubled instead of eGFR decreased by 40%. No new adverse events were observed in the study.

"Diabetes nephropathy remains the most common cause of end-stage renal disease worldwide, highlighting the need for further exploration of potential renal protection of SGLT2 inhibitors," said Dr. George Bakris, professor and director of the Center for Integrated Hypertension at the University of Chicago School of Medicine. The results of the analysis add a wealth of evidence that canagliflozin potentially improves kidney outcomes in millions of patients with type 2 diabetes, a benefit that can be observed in patients with preserved and reduced renal function."
“Although about one-third of people with diabetes have diabetic nephropathy, there is no significant improvement in treatment,†said Dr. James F. List, director of cardiovascular and metabolic global treatment at Janssen R&D. “We may be diabetic for canagliflozin. Nervous patients are encouraged by the much-needed benefits and look forward to further enhancing this insight in the complete patient recruitment and the first dedicated SGLT2 inhibitor test CREDENCE to assess renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy."
Reference materials:
[1] US FDA Approves BYDUREON for Use with Basal Insulin in Patients with Type 2 Diabetes with Inadequate Glycemic Control
[2] ARKAY Therapeutics Submits Investigational New Drug (IND) Application for RK-01, a First-in Class 'Beta-cell-centric' Drug Combination Product for Type 2 Diabetes
[3] FDA Permits Marketing of IDx-DR for Automated Detection of Diabetic Retinopathy in Primary Care
[4] INVOKANA® (canagliflozin) Demonstrated Significant Renal Protective Benefits in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease
Original Title: Summary of Recent Progress in Diabetes Research (No. 55)
Patient Monitors,Multi-Parameter Patient Monitor,Portable Patient Monitor,Multi Parameter Vital Monitor
Medton Medical , https://www.medtonmedical.com