Hydrophilic Interaction Chromatography (HILIC) uses an eluent similar to reversed-phase liquid chromatography (RPLC) to provide suitable retention and separation alternatives for hydrophilic or polar to very polar analytes. (Appropriate retention and separation provide an alternative).
RPLC is characterized by little or no retention, so the retention of such hydrophilic analytes (analytes with a log P value of about zero or less) is a challenge (these hydrophilic analytes ( The retention of analytes of the type with log P values ​​of about zero or less is a challenge for RPLC because these analytes have little or no retention by reversed-phase chromatography.
The ACE HILIC column provides an alternative to RPLC for hydrophilic analytes without the use of additives such as ion-pairing reagents.
For many years, it has been common to use high organic content eluents in combination with aqueous components (high organic content eluents combined with aqueous components are common HILIC methods), but the term HILIC was first used at 20 The 1990s [1] was first proposed to represent the organic solvent-rich eluent used for the retention and separation of polar analytes (described as organic solvent-rich eluents used to retain and separate polar analytes).
As a technology, HILIC has been applied to almost all chromatographic applications and has proven to be effective for clinical analysis, food and beverage applications, as well as the pharmaceutical industry and proteomics, where polar to (to) poles The retention and separation of very strong analytes remains a challenge.
The main advantages of HILIC used in chromatography
HILIC polarity to be highly polar analytes carried out (providing) Reserved
As a general practical rule of thumb, if the analyte is eluted before caffeine in RPLC mode (log P~zero (approximately 0)), it may be more suitable for the HILIC separation mode.
If the analyte elutes after caffeine in RPLC mode, it is more suitable for RPLC.
Figure 1 shows (represented) an RPLC gradient chromatogram of a mixture containing glyphosate (log P = -2.39), caffeine (log P = -0.13) and amitriptyline (log P = 4.90).
The top of Figure 1 shows an approximation, and the HILIC and RPLC modes can be run and overlapped in the log P scale (showing an approximate position of the HILIC and RPLC mode operations and overlaps at the Log P scale).
It is clear that the very polar analyte glyphosate will elute near the stigma collapse in RPLC mode and (indicate) it is well suited for HILIC.
RPLC mode retains caffeine (caffeine can be used in RPLC mode), but HILIC can also be used. The overlap between RPLC and HILIC often leads to discussion because both models have an advantage: the choice is usually application-oriented (as determined by existing applications).
Amitriptyline is the most suitable hydrophobic analyte for RPLC (hydrophobic analytes are best suited for RPLC).
If the log P characteristics of the analyte are not known, extensive search gradients running in the RPLC mode (shown in Figure 1) can often help determine the (analyte) hydrophilicity/hydrophobicity of the approximate analyte while indicating the most appropriate Separation mode.
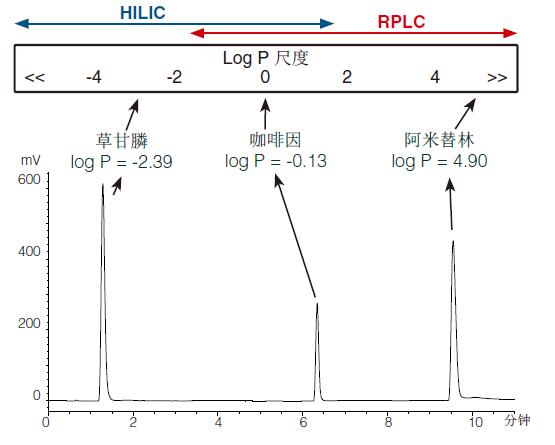
figure 1
HILIC and RPLC operating range and overlapping actual demonstration columns: ACE 2 C18, 100 x 3.0 mm
Mobile phase: A = 10 mM ammonium formate, pH 3.0 (aq) B = 10 mM ammonium formate, pH 3.0 in MeCN: H2O (90:10 v/v)
Gradient: 5-100% B (10 minutes)
Detection: ELSD
Flow rate: 0.4 mL/min
Temperature: 30oC
Injection: 10 μL
Analysis of Chromaster (with VWR ELSD90) using VWR-Hitachi
HILIC is RPLC provides alternative
The second advantage of HILIC is that it typically provides complementary or orthogonal selection for RPLC, as demonstrated in Figure 2.
In the HILIC mode (Fig. 2b), the elution profile (elution) of the mixture of the six polar analytes was exactly the opposite of the same mixture using RPLC analysis, demonstrating that HILIC has orthogonal selectivity.
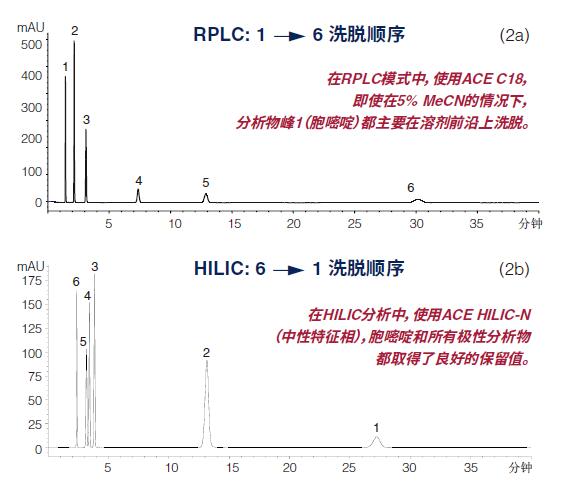
figure 2
Orthogonal selectivity between RP and HILIC (a) Column: ACE 5 C18, 150 x 4.6 mm
Mobile phase: 0.1% acetic acid (dissolved in acetonitrile/water) (5:95 v/v)
(b) Column: ACE 5 HILIC-N, 150 x 4.6 mm
Mobile phase: 0.1% acetic acid (dissolved in acetonitrile/water) (95:5 v/v)
Flow rate: 1 mL/min
Temperature: 22 °C
Detection: UV, 254 nm
Injection: 5 μL
sample:
1) Cytosine
2) Hypoxanthine
3) Thymine
4) Theobromine
5) Theophylline
6) Caffeine
Selectivity is the key to analyte precipitation (resolution) in chromatographic analysis, so HILIC can be used to maximize the selectivity of polar to very polar analytes (for polar to strong polar compounds, HILIC can be maximized) Selective).
In method development, the use of selectivity is the first step in understanding the retention behavior of an analyte, and it is also an effective tool to increase the likelihood of all species within the sample (sample) being observed.
The different and complex HILIC multi-mode retention mechanism provides an ideal complementary selective separation mode for RPLC.
HILIC eluent used has a high content of machine
The third advantage stems from the high organic content eluent used in the HILIC mode.
The results show that compared with RPLC, its fast diffusion coefficient can reach twice that of RPLC, which can strengthen the mass transfer and reduce the effect of C term on the Fandamt equation (reducing the Phase II of Fantem's equation) [2]. .
However, the mobile phase in HILIC must contain at least 3% aqueous material to form (as needed) an aqueous adsorbate layer around the stationary phase particles for dispersion (distribution). The aprotic organic solvent acetonitrile is commonly used as the weaker solvent in HILIC.
In addition, HILIC eluents are also helpful for MS detection [3].
Very high organic solvent content, ideal for low surface tension (high organic solvent content provides an ideal low surface tension) and high volatility liquid flow for MS sources to improve desolvation and ion formation Conditions, thus enhancing signal response.
HILIC separation typically provide low back pressure
Typically, the column (and system (system)) back pressure in HILIC mode is lower than the water-organic RPLC eluent (especially when the most commonly used acetonitrile is used).
This gives chromatographers a choice: with higher flow rates and even multiple sets of columns combined (in series), smaller, more efficient particles can be used, increasing plate count (theoretical plates) Or peak capacity, while the back pressure [4,5] is also moderate (in the case of suitable back pressure, the plate count (the number of theoretical plates) or peak capacity [4,5]).
The main disadvantage of HILIC used in chromatography
Stability of the HILIC method
This is a HILIC-related (related to HILIC) topic that is often discussed and reported.
Many problems can be solved by using a sufficiently balanced HILIC column before use.
If the HILIC method contains a gradient (gradient elution), then sufficient balance is required between the injections.
Sample solubility
Due to its nature, polar to very polar analytes are difficult to dissolve in organic-rich solvents and remain in a dissolved state (difficult to dissolve and remain in a solution in a solution containing large amounts of organic matter).
Small Safe,Smart Safe,Electronic Safe,Portable Safe Box
Hebei Yingbo Safe Boxes Co.,Ltd , https://www.ybsafebox.com