In recent years, with the development of science and technology, PD-1 and CAR-T cell therapy have achieved a lot of achievements, which has opened up another life path for cancer patients. At the same time, in the field of immunotherapy, there is another research and development that is also worthy of attention. It is a bispecific antibody.
Bispecific antibody (BsAb), also known as bifunctional antibody, is a kind of artificial engineering that can simultaneously recognize and bind two different antigens and epitopes and block two different signaling pathways to play their roles. antibody. It does not exist in the natural state and can only be prepared manually.
As an emerging antibody class, BsAb is of great significance for the immunotherapy of cancer and is regarded as a prospective candidate for cancer and cancer treatment. A large number of bispecific antibody drugs are currently in clinical research and many clinical trials are ongoing.
So, what exactly is a bispecific antibody? And look down.
The early predecessor of modern BsAb was developed in the 1980s. The first generation BsAbs were developed using chemical coupling methods or four-color techniques involving somatic hybridization of two antibody-secreting hybridoma cells. However, these methods are limited by their immunogenicity or their manufacturing complexity. Recently, the development of BsAb has made new progress because new molecular, structural and computational biology technologies have contributed to the innovation of the BsAb platform. Therefore, there are now many new forms of bispecific antibodies (approximately 100) available (Figure 1), and there are currently more than 50 BsAbs involved in multiple therapeutic indications at different stages of clinical development, including tumors, autoimmunity Sexual, inflammatory, infectious and cardiovascular diseases (Figure 2).
Â
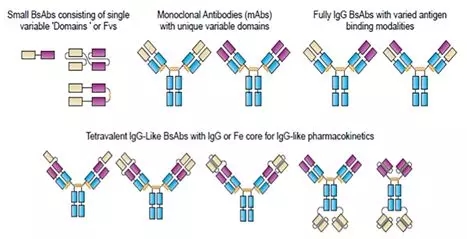
Fig. 1. Schematic diagram of relevant bispecific antibody structures [1] Â
Broadly speaking, BsAbs can be divided into three major structural categories: (1) antibody fragments lacking the Fc domain (eg, diabody and tandem single-chain variable fragments [scFvs]); (2) antibody fragments or Alternative scaffold proteins fused to antibodies, antibody Fc regions or human serum albumin to enhance their pharmacokinetic (PK) properties and/or effectiveness (eg, dual-variable immunoglobulin [DVD-Igs] and IgG-scFvs) (3) Complete IgG BsAbs maintain the architecture of native IgG, which may help to avoid non-natural problems associated with antibody fragments. Each structure has its own unique advantages, and some of the engineered BsAbs used to treat a variety of autoimmune and inflammatory diseases are in clinical development (Figure 2) .
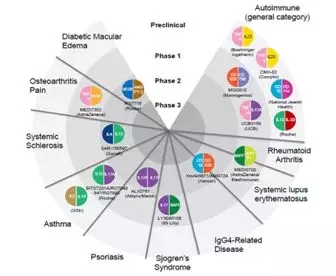
Fig. 2. Bispecific antibodies currently in preclinical or early-phase trials for autoimmune and inflammatory conditions [2]
Targeting two (or more) effective pathways to enhance therapeutic efficacy is an attractive and logical application of BsAbs in the treatment of cancer and cancer. Therapeutic strategies for well-designed BsAbs include selective targeting of specific immune cell types, colocalization of cell surface receptors and cross-linking to induce new signaling pathways, or redirecting the immune system to target pathogenic cells Types of. The details are as follows:
1. Target multiple receptors to selectively affect specific cell types
Bispecific antibodies can be designed to interact simultaneously with two cell membrane-associated receptors that are cell type specific. This ability can be used to selectively modulate signaling in pathogenic immune cell types. By selectively binding to cells expressing both target proteins, the BsAb can be modulated to achieve a higher selectivity for the desired target cell type and/or activity.
2. New signal transduction through colocalization of receptors and supercrosslinking of immunoreceptors
Another novel function of BsAb that can be used for therapy is related to its ability to cross-link immunoreceptors that enhance signal transduction or replication of target aggregation that occurs during immunomodulation. For example, BsAbs or bispecific fusion proteins are designed to introduce regulatory receptors directly into the immune signaling complex, thereby affecting immune cell signaling or differentiation.
3. Redirect T cell targeting pathogenic cell types
One of the most well-known applications of BsAb is to redirect effector cells (such as T cells, natural killer cells or monocytes/neutrophils) to destroy malignant cells, which can make antigen-expressing cells significantly at the target receptor level. Target receptor levels required for antibody-dependent or complement-dependent cytotoxicity.
Â
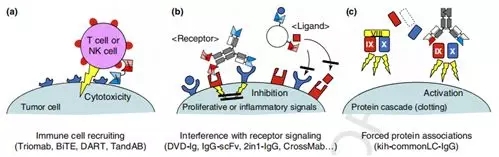
Fig. 3.Mode of action of therapeutic bispecifific antibodies (bsAbs). (a) Recruiting of T cells or natural killer (NK) cells to tumors is achieved by entities that bind to tumor cell surface antigens as well as to immune cells. Are Tribs in various formats have been developed for this mode of action, such as DVD, are TrioMabs (catumaxomab), BiTEs (blinatumomab), DARTs, and TandAbs. (b) Interference withreceptorsignaling is achieved by binding cell surface receptors or to their cognate ligands. -Igs, DAFs, 2-in1-IgG, Tv-IgGs, and CrossMabs. (c) One exciting 'unusual' application of bsAbsis antibody-mediated forced assembly of the coagulation Xase complex. A heterodimeric common light chain IgG connects FXIa and FX And Bounds FVIII deficiency. Abbreviations: BiTE, bispecific T cell engager; DAF, dual-action Fab; DART, Dual affinity retargeting; DNL, ​​dock-and-lock; DVD-Ig, dual variable domain immunoglobulins; FX, Factor X; HSA, human serum albumin; Ig, immunoglobulin; kih, k Nobs into holes; Tv, tetravalent. [3]
Â
Bispecific antibodies represent the next generation of therapeutic antibodies, providing clinical opportunities to implement complex targeting strategies with the goal of achieving greater efficacy in treating tumors and cancer than existing biologics. Based on the patient's own unique immune characteristics, BsAb is expected to provide new options for personalized treatment of cancer and cancer. Although the use of BsAbs in autoimmune diseases and inflammatory diseases is still in its infancy, recent advances in its engineering have led to the development of more powerful, and in some cases more IgG-like, molecules. Although there are still many challenges, BsAb has broad clinical application prospects and is expected to play an important role in future cancer and cancer treatment.
Ok, this sharing is over, follow up with more exciting content. If you like it, please continue to pay attention!
Â
references:
[1].Froning K, Leaver-Fay A, Wu X, Phan S, Gao L, Huang F, et al. Computational design of a specifific heavy chain/κ light chain interface for expressing fully IgG bispecifific antibodies. Protein Sci2017;26 :2021–38.
[2].Bispecifific antibodies: The next generation of targetedinflflammatory bowel disease therapies
[3]. http://dx.doi.org/10.1016/j.drudis.2015.02.008
Shandong Zhushi Pharmaceutical Group Co.,LTD , https://www.sdzs-medical.com