Brooke M. Koshel and Sean M. McCarthy
Waters Corporation (Milford, MA, USA)
Key words
Method Conversion, ACQUITY Arc, IEX, Monoclonal Antibody
Application advantage
â– Seamlessly convert methods to the ACQUITY Arc system without changing method parameters.
â– Easily reproduce HPLC or UHPLC separations using Arc Multi-flow pathTM technology.
Waters Solutions
ACQUITY ® ArcTM System
2489 UV/Vis detector
Empower ® 3 Chromatography Data Software (CDS)
Introduction
Today, many of the best-selling biopharmaceuticals are protein drugs. Unlike controlled chemical processes for the synthesis of small molecule drugs, biologics are produced using live cells and are therefore more susceptible to variability. To ensure product quality and safety, the entire life cycle of biopharmaceuticals often requires the application of multiple analytical techniques. Ion exchange chromatography (IEX) is a commonly used charge-variant monitoring technique that reflects the changes that biopharmaceuticals undergo at different stages of production, such as fermentation, purification, and formulation.
The ACQUITY Arc system is part of the Quaternary LC portfolio and is designed to address the consistency of results that analysts face throughout the product testing process. The ACQUITY Arc system uses Arc Multi-flow path technology to easily reproduce traditional HPLC methods (stream 1) or to convert existing methods into a better performing UHPLC separation method (stream 2). This application note will focus on how to easily reproduce existing monoclonal antibody ion exchange separation methods and achieve reproducible results.
experiment
LC condition
Liquid phase system: equipped with 2489 UV-visible detector ACQUITY Arc system, flow path 1
Agilent 1100 Series HPLC System with Quaternary Pump and DAD Detector Absorption Wavelength: 280 nm
Sampling rate: 20 Hz
Column: Dionex ProPac WCX-10 10 μm, 4.0 mm x 250 mm Column column temperature: 30 °C
Mobile phase A: 0.02 M MES , pH 6.0
Mobile phase B: 0.02 M MES, 0.4 M sodium chloride, pH 6.0
Sample: 1 mg/mL rituximab, dissolved in a solution containing 0.15 M sodium chloride, 0.025 M sodium citrate and 0.06% (v/v) Tween 80, pH 6.5
Sample temperature: 5 °C
Injection volume: 40 μL
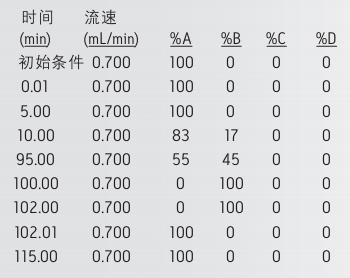
gradient
Data management
Empower ® 3 CDS Software, SR2
Results and discussion
The ACQUITY Arc system easily reproduces existing IEX-HPLC methods with excellent reproducibility
In general, analysts need to monitor charge variants throughout the life cycle of a drug and perform rigorous process control. Therefore, no matter what instrument is used for testing, analysts must determine the differences between systems to ensure product quality. Ideally, any system used must be able to provide consistent results consistent with product analysis guidelines. To investigate the feasibility of converting cation exchange methods from traditional instruments to modern LC platforms, we analyzed rituximab (a monoclonal antibody) using the Agilent 1100 Series HPLC system and analyzed the results with the ACQUITY Arc system. The results were compared. The method used was selected from the USP Drug Standards Set 1 and although the method has now been discontinued, it is still a representative method in the pharmaceutical industry.
We did not make any modifications to the above method and applied it directly to the Agilent 1100 Series HPLC system and the ACQUITY Arc system (Figure 1). As shown, the chromatographic performance of the two systems is almost identical. As shown in the inset of Figure 1, the method successfully maintains the same selectivity and resolution when switching between the two platforms. Table 1 lists the relative retention times of the main peaks obtained by data processing, as well as the relative retention times of other acidic and basic variants. Although there is some difference between the retention times of the analytes on the two systems, it is worth noting that the relative retention times of all major chromatographic peaks are essentially identical.
We can determine whether the analytical method meets the system suitability criteria by calculating multiple parameters. Separation is one of them. The separation of the main peak (MP) of rituximab from the peak of lysine variant (1B) is not less than 1.5 according to USP Drug Standard Set 1. The average resolution of the Agilent 1100 Series HPLC system after 5 injections. The average resolution of the ACQUITY Arc system is 1.09 ± 0.02, and both systems are compliant. To ensure product consistency, it is not only necessary to have reproducibility between systems, but also to ensure reproducibility within the same instrument. Figure 2 shows the overlay chromatogram obtained on the ACQUITY Arc system using the above method conditions for 5 replicate injections of rituximab. Each chromatogram almost completely overlaps throughout the run time range. The magnified chromatogram shown in the illustration further confirms this conclusion.
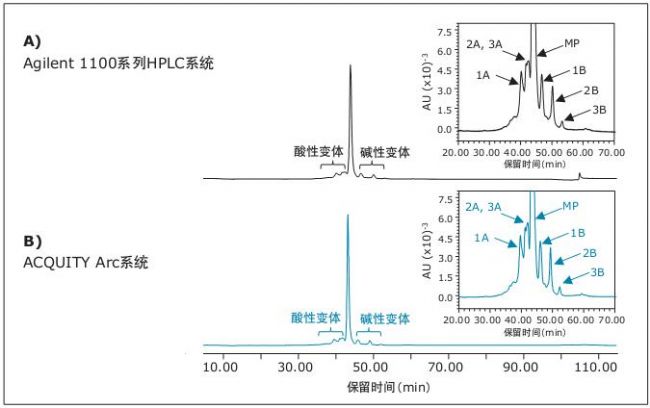
Figure 1. IEX acquisition data comparison using A) Agilent 1100 Series HPLC System and B) ACQUITY Arc System. The inset shows the separation of acidic and basic variants. The main peak of rituximab is indicated as MP. Other acidic (1A, 2A, 3A) and basic (1B, 2B, 3B) residues were labeled accordingly and their retention times are listed in Table 1.
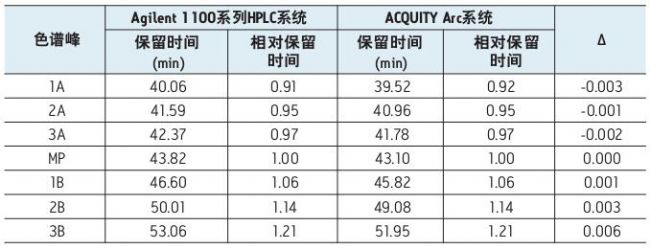
Table 1. Comparison of retention times for IEX analysis on Agilent 1100 Series HPLC systems and ACQUITY Arc systems. The peaks of the acidic and basic components are shown in Figure 1. A ∆ value indicates the difference between the relative retention times of the two systems. All results are the average of 5 injections.
Table 2 lists the results of quantitative analysis of the data in Figure 2, including the peak area percentages of the main peak, the acidic variant, and the basic variant, and the data obtained by injecting these values ​​with the Agilent 1100 Series HPLC system for 5 injections. Compare the situation. Overall, the data obtained by the two systems is comparable. The peak area differences of the main peak, the acidic variant and the basic variant obtained by the two systems were 0.53%, 0.38% and 0.90%, respectively.
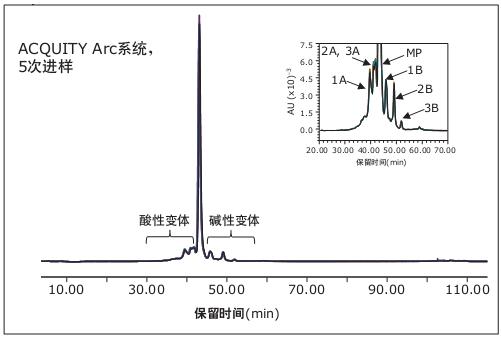
Figure 2. Overlay of IEX chromatograms for 5 injections of the ACQUITY Arc system. All peaks eluting prior to the main peak are acidic variants, while all peaks eluting after the main peak are alkaline variants. From the illustrations we can identify three acidic variants (1A, 2A, 3A) and three basic variants (1B, 2B, 3B). Table 2 lists the total acidic variants and total alkaline variants. Summary results.
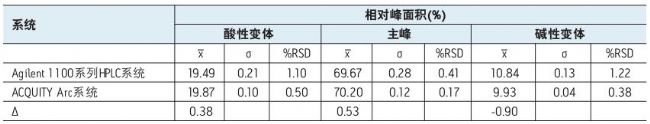
Table 2. Quantitative comparison of IEX data collected by the Agilent 1100 Series HPLC System and the ACQUITY Arc System. Acidic and basic variants include all substances that elute prior to the main peak and elute after the main peak. The ∆ value indicates the difference in peak areas obtained by the two systems. All results are the average of 5 injections.
in conclusion
With Arc Multi-flow path technology, users can use the ACQUITY Arc system to simulate traditional HPLC methods or update analytical methods to UHPLC separation methods. This application note confirms that the IEX method can be easily converted from the Agilent 1100 Series HPLC system to the ACQUITY Arc system (Stream 1). With all the method parameters unchanged, we obtained a chromatogram with almost identical retention times. In addition, the results of five replicate injections on the ACQUITY Arc system showed extremely high reproducibility.
references
1. USPharmacopeial Convention, http:// (accessed March 2015).
Waters China Ltd. Waters Technology (Shanghai) Co., Ltd. Beijing: 010 - 5209 3866
Shanghai: 021 - 6156 2666
Guangzhou: 020 - 2829 5999
Chengdu: 028 - 6765 3588
Hong Kong: 852 - 2964 1800
Free after-sales service hotline: 800 (400) 820 2676
ALUMINUM ROLLING WALKER WITH SEAT AND BACKREST folds easily for storage and transportation. No tools needed and easy to assemble. Made of durable but lightweight aluminum that can hold up to 300 pounds
ROLLATOR WALKER WITH SEAT helps increase mobility with 8 inch PVC wheels with bicycle style loop-lock handbrakes.
Rollator Walker NYLON SEAT is built in with backrest and adjustable handle heights to ensure optimized fit and comfort making it the perfect walkers for seniors.
Rollator For elderly,Aluminum Rollator Walker,disabled Rollator,Medical Rollator,Rollator
Foshan Oscar Medical Instrument CO.,LTD , https://www.fsoscarmed.com